Fusion protein and application thereof to treating clostridium difficile related diseases
A technology of Clostridium difficile and fusion protein, which is applied in the field of fusion protein and its application in the treatment of Clostridium difficile-related diseases, and can solve the problems of repeated illness of patients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] Example 1. Different protein compositions and functional verification thereof
[0056] 1. Preparation of different protein compositions
[0057] 1. Genes encoding proteins
[0058] The amino acid sequence of TcdA is sequence 2 in the sequence listing, and the nucleotide sequence of its coding gene is sequence 1;
[0059] The amino acid sequence of TcdB is sequence 4 in the sequence listing, and the nucleotide sequence of its coding gene is sequence 3;
[0060] The amino acid sequence of BclA3 is sequence 6 in the sequence listing, and the nucleotide sequence of its coding gene is sequence 5;
[0061] The amino acid sequence of CD0873 is sequence 8 in the sequence listing, and the nucleotide sequence of its coding gene is sequence 7;
[0062] The amino acid sequence of FliD is sequence 10 in the sequence listing, and the nucleotide sequence of its coding gene is sequence 9;
[0063] The nucleotide sequences of the coding genes of TcdA, TcdB, BclA3, CD0873 and FliD we...
Embodiment 2
[0107] Embodiment 2, different fusion protein composition and application thereof
[0108] 1. Preparation of fusion protein composition
[0109] 1. Preparation of fusion protein
[0110] 1) The fusion protein TcdA-TcdB is sequentially composed of TcdA, connecting peptide (G 3 S 1 ) 3 , TcdB composition, its amino acid sequence is followed by TcdA amino acid sequence from N, (G 3 S 1 ) 3 Amino acid sequence, TcdB amino acid sequence composition; The nucleotide sequence of the coding gene of fusion protein TcdA-TcdB is followed by the nucleotide sequence of TcdA coding gene, (G 3 S 1 ) 3 Nucleotide sequence of coding gene, composition of nucleotide sequence of TcdB coding gene;
[0111] 2) The fusion protein TcdB-TcdA is sequentially composed of TcdB, connecting peptide (G 3 S 1 ) 3 , TcdA, its amino acid sequence from N is followed by TcdB amino acid sequence, (G 3 S 1 ) 3 Amino acid sequence, TcdA amino acid sequence composition; The nucleotide sequence of the c...
Embodiment 3
[0147] Embodiment 3, the immune protection of protein composition and fusion protein composition
[0148] 1. Immunoprotective properties of protein composition and fusion protein composition
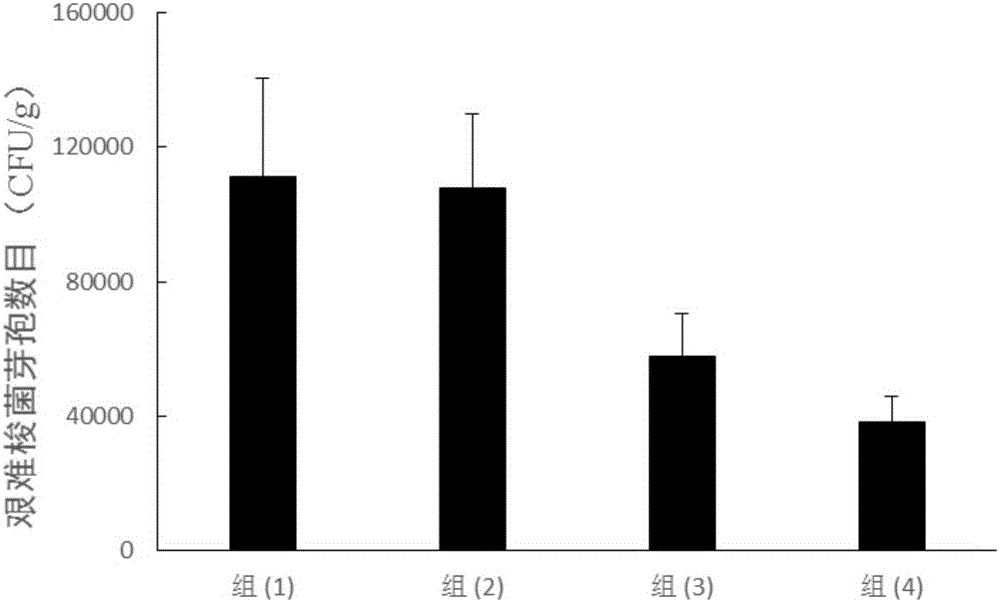
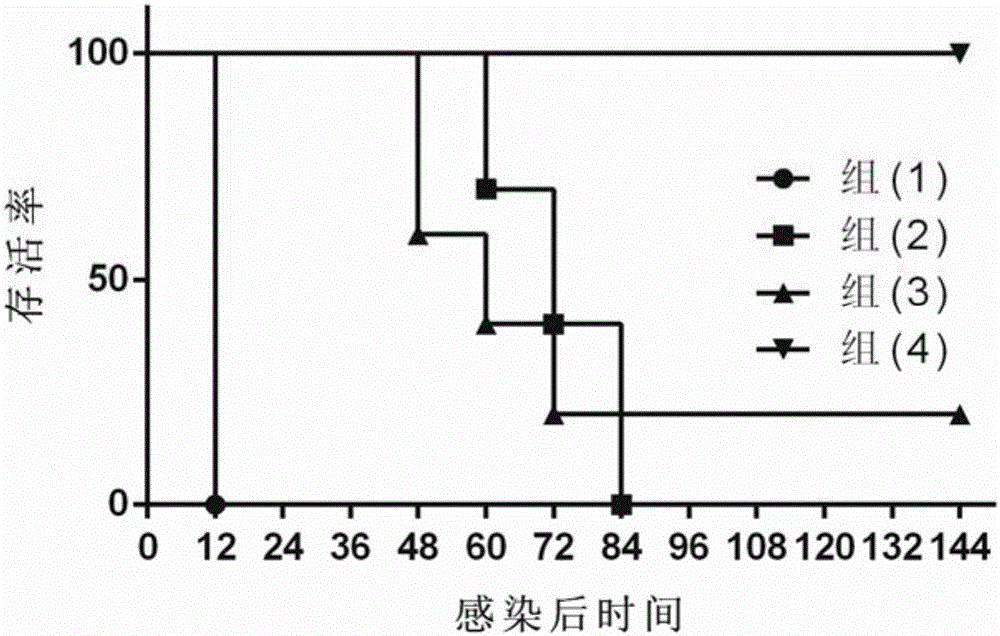
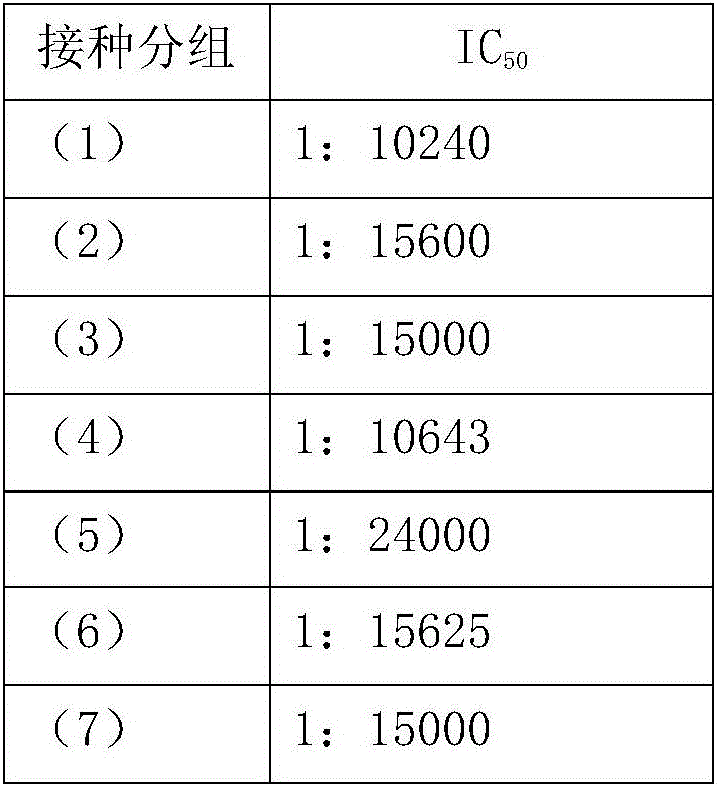
[0149] 6-week-old BALB / C mice were randomly divided into 4 groups, and the following intramuscular injections were immunized respectively: (1) normal saline group; (2) the protein composition TcdA+TcdB 10 μg of embodiment 1; (3) the protein composition of embodiment 1 Protein composition BclA3+CD0873 10 μg; (4) fusion protein composition TcdB-TcdA+BclA3-CD0873 10 μg in Example 2; 100 μl each. The left leg muscle was injected on the 0th day, and the right leg muscle was injected on the 14th day; on the 28th day, 100 CFU of Clostridium difficile ATCC 43255 spores were administered orally for infection. Seven days before infection, 0.5 mg / ml cefoperazone was added to the drinking water of the mice (freshly prepared every two days, and the mice drank water freely); after 5 days of treatment...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com