A hybridoma cell line secreting anti-human il-37b monoclonal antibody, anti-human il-37b monoclonal antibody and application thereof
A hybridoma cell line and monoclonal antibody technology, applied in the field of genetic engineering, can solve the problems of limited application scope, insufficient to meet experimental research, few types of monoclonal antibodies, etc., and achieve the effect of high specificity and good passaging ability.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
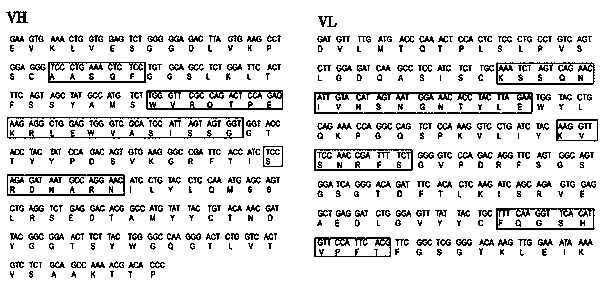
[0048] Example 1: Preparation, identification and purification of antibodies
[0049] 1. Immunogen preparation and animal immunization
[0050] The plasmid containing the IL-37b gene fragment stored in the laboratory was used to transform into Escherichia coli, and IPTG induced Escherichia coli to express a large amount of the target protein. After the Escherichia coli was sonicated, it was purified using a nickel column to obtain soluble IL-37b protein. Two 6-week-old female BALB / c mice were prepared, and the mice were immunized with human IL-37b protein according to the scheme in Table 1. The mice were immunized for the first time, and the second immunization was carried out 14 days later. After the first immunization, each mouse received 150 μg of purified recombinant IL-37b emulsified with Freund's incomplete adjuvant three times by intraperitoneal route at weekly intervals. Seven days after the fourth immunization, blood was collected from the mice via the tail vein and ...
Embodiment 2
[0090] Example 2: Identification and preservation of hybridoma cells
[0091] The preservation information of the hybridoma cell line of the present invention is:
[0092] Deposit unit: China Center for Type Culture Collection; address: Wuhan University; date of deposit: May 2016; name: hybridoma cell line 1C6; deposit number CCTCCNO: C201683.
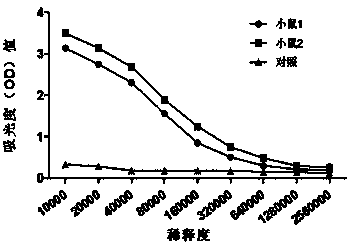
[0093] 1. Stability analysis of antibody secretion ability of different generations of hybridoma cell clones
[0094] Firstly, passage the 1C6 hybridoma cells, and take the first generation, second generation, fifth generation, seventh generation, and tenth generation hybridomas (the initial cell density is that the cells are fully covered at the bottom of the culture flask) after two days of culture The supernatant sample of the cell secretion was identified by ELISA, and the supernatant sample of each generation of cells was used as the primary antibody to obtain the IL-37b prokaryotic purified protein plate, and the ELISA was perfo...
Embodiment 3
[0097] Example 3: Application of anti-human IL-37b monoclonal antibody
[0098] 1. Mouse anti-human IL-37b monoclonal antibody applied to WB
[0099] (1) Reagents and preparation
[0100] 1. Electrode buffer (prepared with 1× electrode buffer):
[0101] Tris base 3.03g, glycine 18.8g, SDS 1.0g, double distilled water to adjust the volume to 1L, pH8.3;
[0102] 2. Transfer buffer: glycine 2.9g, Tris base 5.8g, SDS 0.37g, methanol 200ml, add ddH 2 O is fixed to 1000ml, pH8.3;
[0103] 3. TBST (Tris-Hcl, Nacl) buffer: 6g Tris base, 9g Nacl, 500ml double distilled water, Hcl pH7.5, constant volume 1L, add 1ml Tween-20;
[0104] 4. Blocking solution: 8% skimmed milk powder;
[0105] 5. 30% acrylamide storage solution: 29.2g acrylamide, 0.8g methylenebisacrylamide, add double distilled water to 100ml;
[0106] 6. Stacking gel buffer: 1mol / L Tris base, pH6.8;
[0107] 7. Separating gel buffer: 1mol / L Tris base, pH8.8;
[0108] All the above reagents were prepared with double ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com