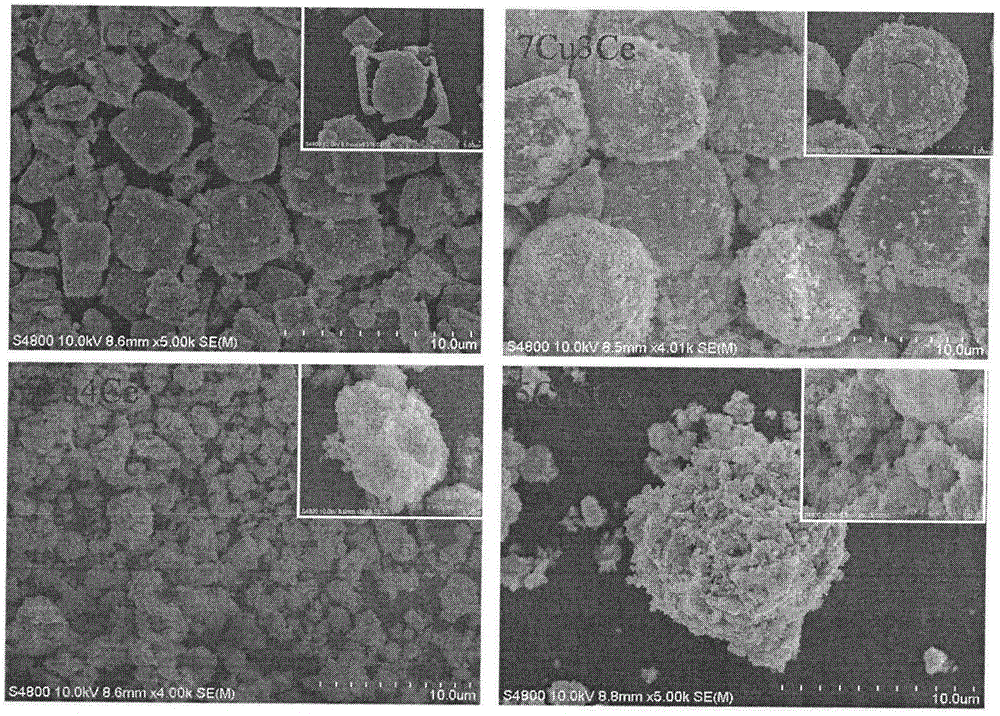
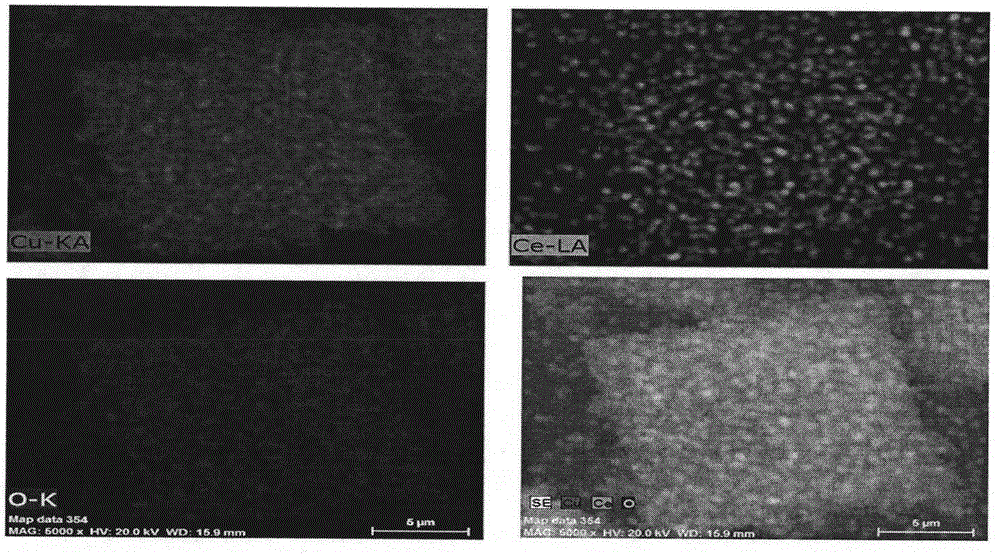
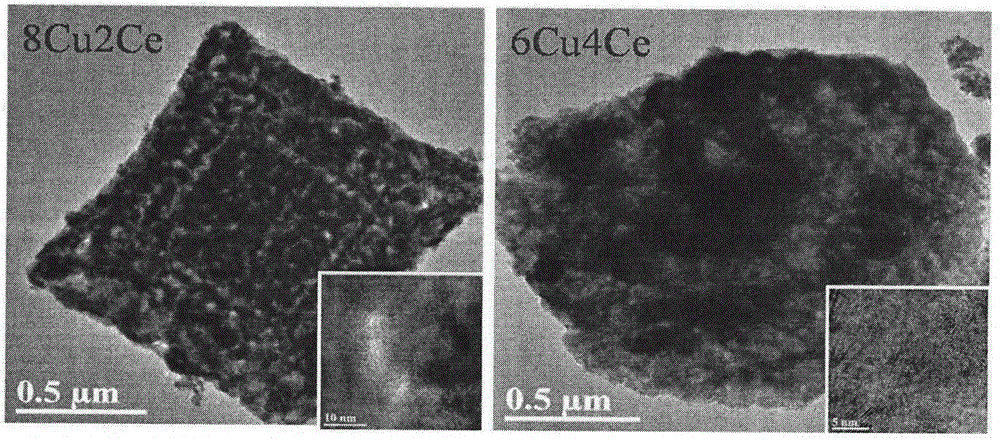
Preparation method for one-step synthetic multilayer nanometer box-shaped CuO-CeO2 catalyst for CO preferential oxidation reaction in hydrogen-rich gas
An oxidation reaction, nano-box technology, applied in the direction of metal/metal oxide/metal hydroxide catalyst, physical/chemical process catalyst, chemical instrument and method, etc., can solve the problem of Pt electrode poisoning, etc., and achieve the goal of widening the complete conversion of CO Window, the effect of improving catalyst performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Embodiment 1 molar ratio is the test result of Cu: Ce=8: 2:
[0038] At 75°C, the conversion rate of CO is 8.43%, and the selectivity is 100%;
[0039] At 95°C, the conversion rate of CO is 8.31%, and the selectivity is 100%;
[0040] At 115°C, the conversion rate of CO is 17.67%, and the selectivity is 100%;
[0041] At 135°C, the conversion rate of CO is 40.81%, and the selectivity is 78.29%;
[0042] At 155°C, the conversion rate of CO is 78.21%, and the selectivity is 53.64%.
[0043]At 175°C, the conversion rate of CO is 82.55%, and the selectivity is 48.11%.
[0044] At 195°C, the conversion rate of CO is 55.61%, and the selectivity is 32.40%.
[0045] At 215°C, the conversion rate of CO is 35.01%, and the selectivity is 20.49%.
Embodiment 2
[0046] Embodiment 2 molar ratio is the test result of Cu: Ce=7: 3
[0047] At 75°C, the conversion of CO is 3.76%, and the selectivity is 100%.
[0048] At 95°C, the conversion rate of CO is 9.43%, and the selectivity is 100%;
[0049] At 115°C, the conversion rate of CO is 23.02%, and the selectivity is 92.63%;
[0050] At 135°C, the conversion rate of CO is 54.87%, and the selectivity is 79.70%;
[0051] At 155°C, the conversion rate of CO is 88.48%, and the selectivity is 51.72%;
[0052] At 175°C, the conversion rate of CO is 79.73%, and the selectivity is 42.56%.
[0053] At 195°C, the conversion rate of CO is 74.18%, and the selectivity is 39.65%.
[0054] At 215°C, the conversion rate of CO is 54.17%, and the selectivity is 28.69%.
Embodiment 3
[0055] Embodiment 3 molar ratio is the test result of Cu: Ce=6: 4:
[0056] At 75°C, the conversion of CO is 30.33%, and the selectivity is 100%.
[0057] At 95°C, the conversion rate of CO is 74.27%, and the selectivity is 100%;
[0058] At 115°C, the conversion rate of CO is 97.39%, and the selectivity is 95.95%;
[0059] At 135°C, the conversion rate of CO is 100%, and the selectivity is 81.61%;
[0060] At 155°C, the conversion rate of CO is 100%, and the selectivity is 59.75%;
[0061] At 175°C, the CO conversion rate is 94.40%, and the selectivity is 55.72%.
[0062] At 195°C, the conversion rate of CO is 73.52%, and the selectivity is 43.71%.
[0063] At 215°C, the conversion rate of CO is 56.90%, and the selectivity is 33.75%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com