Method for detecting activity of residual trypsin in cell product
A trypsin and detection method technology, applied in the field of protein detection, can solve the problems of unsuitable detection of trypsin activity, etc., and achieve the effects of simple method, improved sensitivity, and increased reaction temperature
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment
[0023] A method for detecting residual trypsin activity in cell products, comprising the following steps:
[0024] (1) Blank experiment: Take 2.5-3.5ml of the substrate solution, add 150-250μl of 0.001mol / L hydrochloric acid solution, mix well, and use it as a blank experiment.
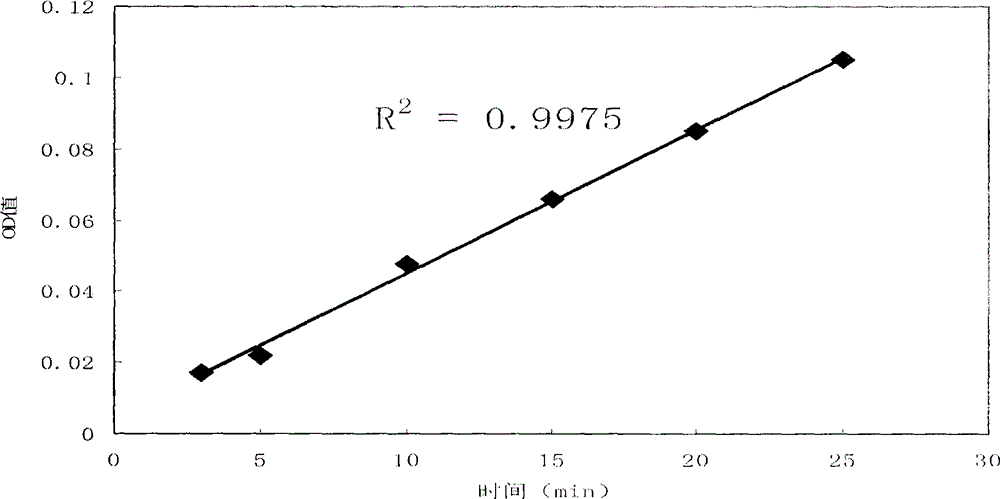
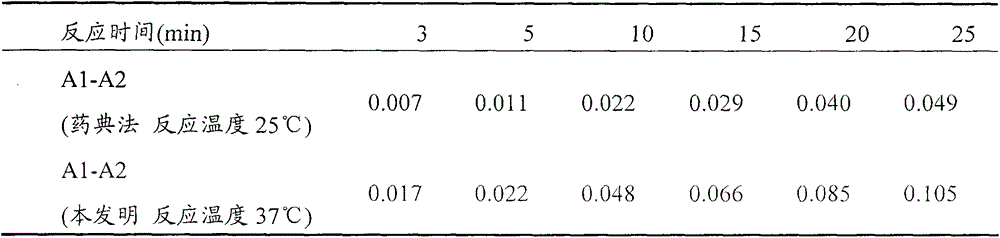
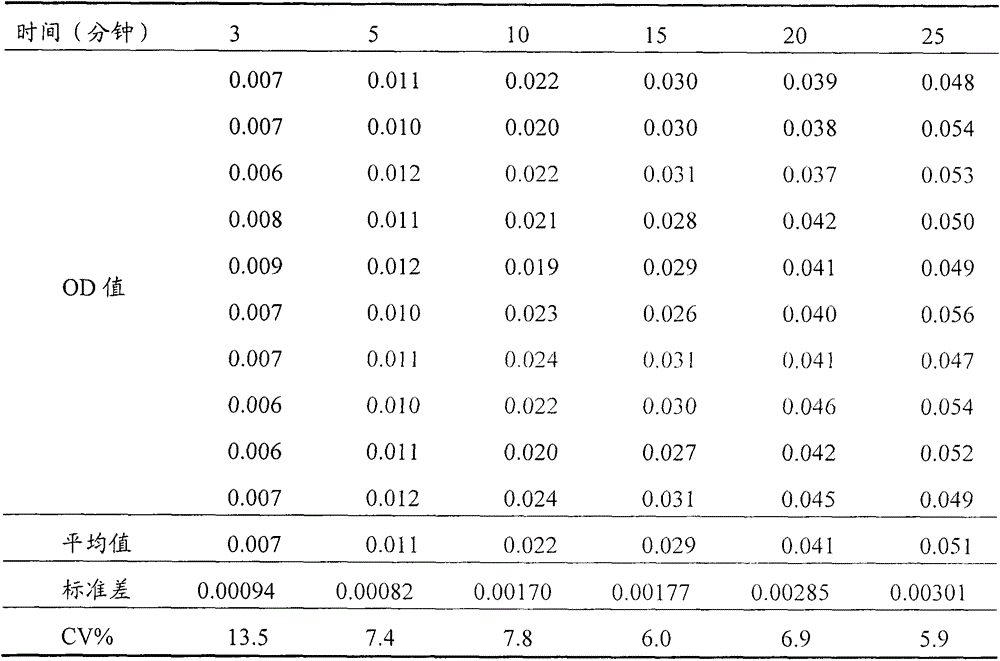
[0025] (2) Determination of residual trypsin activity of cell products: take 100 μl of cell product solution, add 2.5-3.5 ml of substrate solution and 80-120 μl of phosphate buffer solution, immediately time and mix evenly, and measure the absorbance OD value at 253nm A2, while keeping the reaction system at 36-38°C; then measure the absorbance OD value A1 at 253nm at the point of reaction for 25 minutes; A1-A2 represent the residual trypsin activity in the product.
[0026] In order to further optimize the above technical scheme, take 3.0ml of the substrate solution, add 200μl of 0.001mol / L hydrochloric acid solution, mix well, and use it as a blank experiment; the blank experiment is used to offset ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com