Method of detecting distribution of nanoparticles in cells and tissues and application thereof
A detection method and nanoparticle technology, applied in the field of medicine and biology, can solve problems such as fatigue, tediousness, and complicated process, and achieve the effects of saving time, more effective data, and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061] Example 1 Quantitative detection of nanoparticle uptake by cells
[0062] (1) Preparation of fluorescent nanoparticles
[0063] The present invention uses liposomes as the model of nanoparticles. Take HSPC, Chol, and DSPE-PEG2000 stock solutions with a mass ratio of 3:1:1, add DiI at a lipid mass ratio of 1%, prepare liposomes by ethanol injection, hydrate with PBS for 30 minutes, and pass 200nm+ 100nm, 80nm+50nm Nuclepore polycarbonate membranes were passed 13 times each. To prevent fluorescence quenching, the preparation process was protected from light. The particle size of the prepared fluorescent liposomes was characterized by DLS.
[0064] (2) Cell uptake experiment
[0065] Plate after cell counting, incubate nanoparticles with a volume ratio of 1:1 with different media (PBS, albumin solution, pure serum), add cell culture medium, and incubate at 37°C for 2 hours, in which the concentration of albumin is 40mg / ml , the nanoparticle concentration was 1 mg / ml. ...
Embodiment 2
[0069] Example 2 Separation and Detection of Tissue Nanoparticles Methodological Investigation
[0070] (1) Preparation of fluorescent liposomes
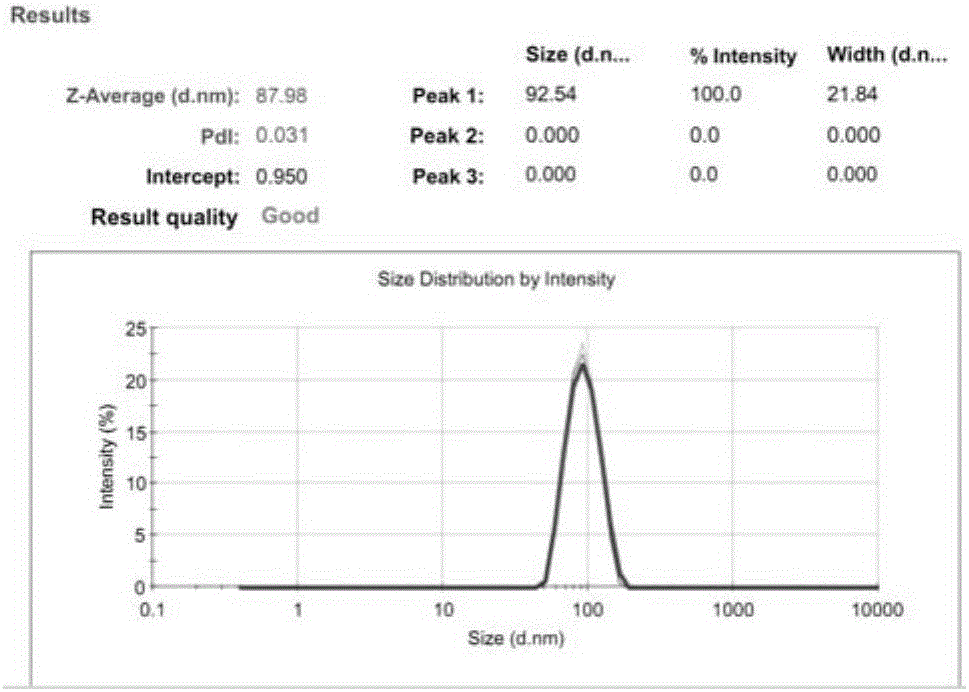
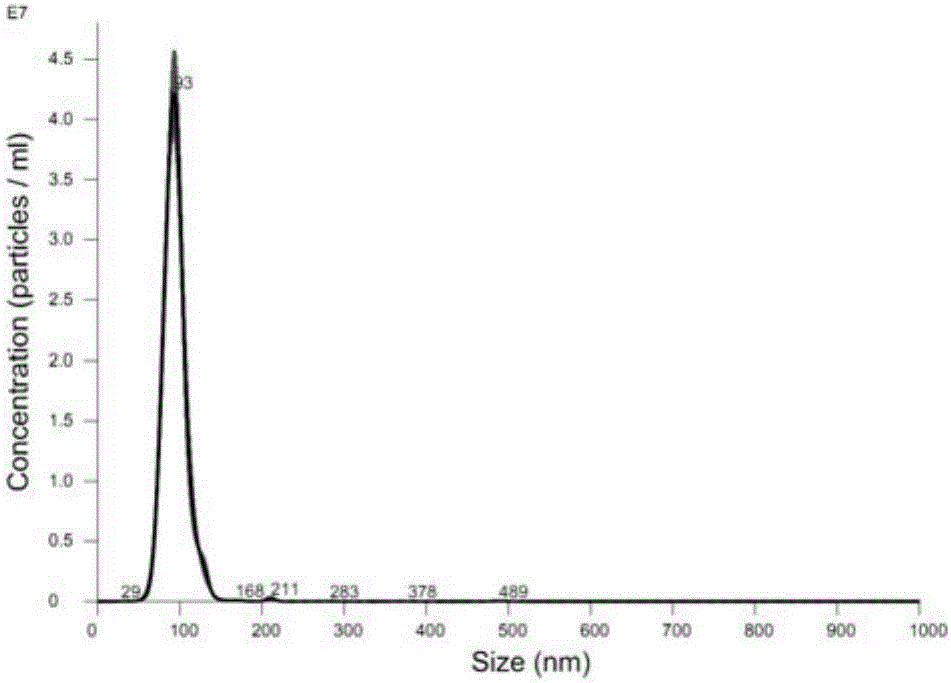
[0071] Take HSPC, Chol, and DSPE-PEG2000 stock solutions with a mass ratio of 3:1:1, add DiI at a lipid mass ratio of 1%, prepare liposomes by ethanol injection, hydrate with PBS for 30 minutes, and pass 200nm+ 100nm, 80nm+50nm Nuclepore polycarbonate membranes were passed 13 times each. In order to prevent fluorescence quenching, the preparation process was protected from light. The particle size of the prepared fluorescent liposomes was characterized by DLS, and the test results are as attached figure 2 shown.
[0072] (2) Isolation of nanoparticles in tissues
[0073] Cut 50mg of tumor tissue into pieces, add 50ul 6mg / ml, 0.6mg / ml, 0.06mg / ml fluorescent liposomes, mix well, add 750ul dispase solution (concentration is 2U / ml, U refers to a protease activity unit) , in a 37°C incubator, statically digest tumor tissue for 4 hou...
Embodiment 3
[0080] Example 3 Distribution detection of nanoparticles in tumors
[0081] (1) Animal experiments
[0082] The nude mice inoculated with tumors were randomly divided into 6 groups, one group was used as a blank control group, and the other 5 groups were injected with fluorescently labeled liposomes in the tail vein, and the mice were killed 2h, 6h, 9h, 12h, and 24h after administration, respectively. , the tumor tissue was obtained after dissection.
[0083] (2) Isolation of nanoparticles from tumor tissue
[0084] Weigh 50mg of tumor tissue at 2h, 6h, 9h, 12h, and 24h respectively, add 750ul dispase solution (concentration is 0.6U / ml, U refers to a protease activity unit), and digest tumor tissue in a 37°C incubator for 4h , then put it into a centrifuge, separate fluorescent liposomes by gradient centrifugation, centrifuge at 300g, 10min to remove intact cells; take the supernatant and centrifuge at 2000g, 10min to remove dead cells, cell debris, platelets, etc.; take the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com