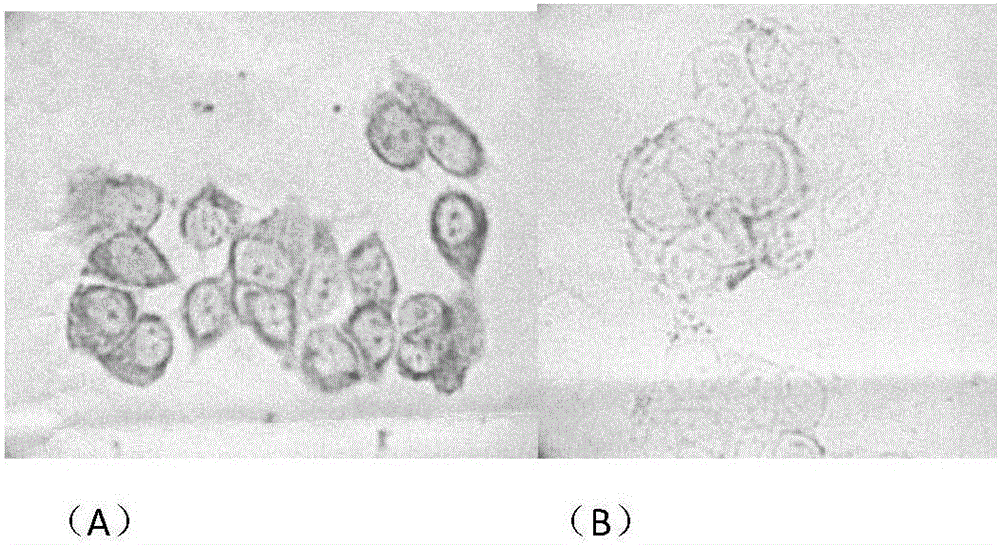
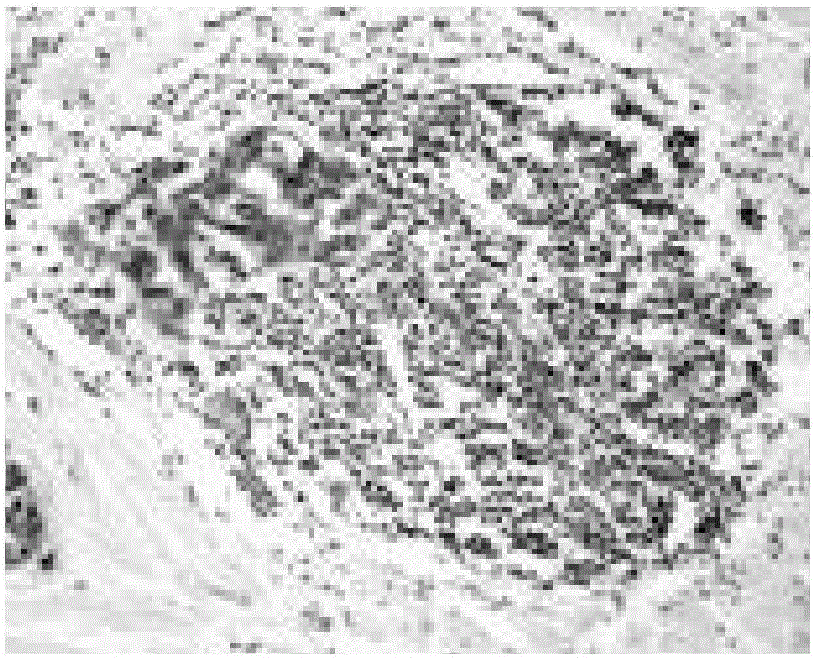
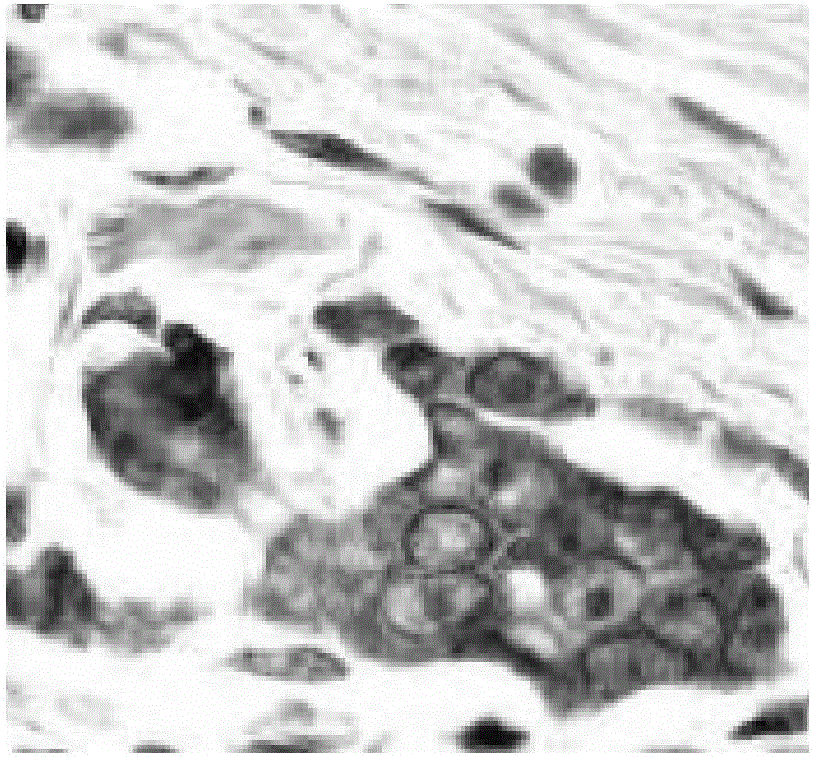
Tumor cell detecting method
A technology for tumor cells and detection methods, applied in biochemical equipment and methods, measuring devices, biological testing, etc., can solve problems such as fluctuations in the effect of cell nucleus staining, difficulty in grasping the time and degree of counterstaining, and affecting observation and judgment of results , achieve the effect of shortening the dyeing time, improving the dyeing effect and easy observation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Example 1: Detection of breast cancer cells
[0044] 1. Preparation of horseradish peroxidase (HRP) marker
[0045] (1) Dissolve 6mg of HRP in 1mL of 0.2mol / L pH4.5 sodium acetate buffer to make HRP solution;
[0046] (2) Add 0.2 mL of 0.1 mol / L sodium periodate solution to the HRP solution, and react in the dark for 30 minutes at room temperature;
[0047] (3) G25 gel column chromatography is used for the reaction solution of step (2), and 0.2mol / L carbonate buffer solution of pH9.5 is eluted, and the activated HRP is collected;
[0048] (4) Add 5 mg of alpha-fetoprotein (AFP) or ovalbumin (OVA) to the activated HRP solution, and react overnight at room temperature in the dark;
[0049] (5) Add 100 μl of freshly prepared 0.1mol / L sodium borohydride solution, and react in the dark for 30 minutes at room temperature;
[0050] (6) The reaction solution in step (5) is chromatographed on a G25 gel column again to collect protein elution peaks;
[0051](7) After concentr...
Embodiment 2
[0092] Example 2: Detection of lymphoma cells
[0093] 1. Preparation of HRP markers
[0094] (1) Dissolve 4mg of HRP in 1mL of 0.1mol / L pH4 sodium acetate buffer to make HRP solution;
[0095] (2) Add 0.1 mL of 0.15 mol / L sodium periodate solution to the HRP solution, and react in the dark for 10 min at room temperature;
[0096] (3) G25 gel column chromatography is used for the reaction solution of step (2), and 0.1mol / L carbonate buffer solution of pH 10 is eluted, and the activated HRP is collected;
[0097] (4) Add 1 mg of AFP or OVA to the activated HRP solution, and react overnight at room temperature in the dark;
[0098] (5) Add 50 μl of freshly prepared 0.05mol / L sodium borohydride solution, and react in the dark for 15 minutes at room temperature;
[0099](6) The reaction solution in step (5) is chromatographed on a G25 gel column again to collect protein elution peaks;
[0100] (7) After concentrating the solution in step (6), purify it with a G200 chromatograp...
Embodiment 3
[0115] Example 3: Detection of prostate cancer cells
[0116] 1. Preparation of HRP markers
[0117] (1) Dissolve 8 mg of HRP in 1 mL of 0.5 mol / L pH5 sodium acetate buffer to make HRP solution;
[0118] (2) Add 0.5 mL of 0.2 mol / L sodium periodate solution to the HRP solution, and react in the dark for 60 minutes at room temperature;
[0119] (3) G25 gel column chromatography is used for the reaction solution of step (2), and 0.5mol / L carbonate buffer solution of pH9 is eluted, and the activated HRP is collected;
[0120] (4) Add 10mg of AFP or OVA into the activated HRP solution, and react overnight at room temperature in the dark;
[0121] (5) Add 200 μl of freshly prepared 0.2mol / L sodium borohydride solution, and react in the dark for 45 minutes at room temperature;
[0122] (6) The reaction solution in step (5) is chromatographed on a G25 gel column again to collect protein elution peaks;
[0123] (7) After concentrating the solution in step (6), purify it with a G20...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com