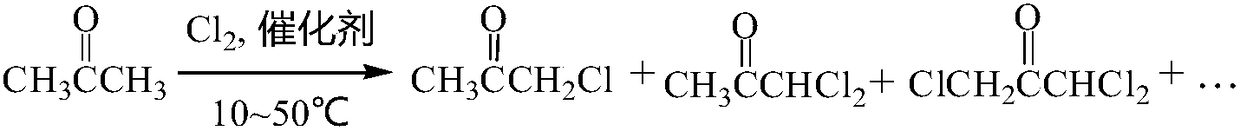A preparation method for improving the synthesis yield of 1,1,3-trichloroacetone
A technology of trichloroacetone and acetone, which is applied in the field of preparation to improve the synthesis yield of 1,1,3-trichloroacetone, can solve the problems of poor selectivity, low yield, long reaction cycle, etc., and achieve firm loading and high chemical properties Stable, easy-to-storage effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Example 1: Add 232.0g (4.0mol) of ultra-pure acetone to a 1000mL reaction flask, slowly add 11.6g of loaded polyamine catalysts under stirring, stir evenly and raise the temperature with a water bath, and wait for the reaction flask to When the temperature is stable at 25°C, start to pass chlorine gas into the reaction bottle for chlorination reaction. The speed of chlorine gas flow is 6.0L / h in the first 1~2h of the reaction, and the speed of 2~6h in the middle stage is 30.0L / h. In the later period of 6-20h, the rate of chlorine gas flow is 6.0L / h. After the chlorine reaction is completed, keep the temperature in the reaction bottle constant and continue to stir for 1-2 hours. After the heat preservation reaction, keep the temperature in the reaction bottle constant, add a certain amount of special solvent into the reaction bottle, and keep stirring for 1 hour. After the stirring was completed, the temperature of the obtained mixture was lowered to 10° C. with ice wat...
Embodiment 2
[0022] Example 2: Add 232.0 g (4.0 mol) of ultra-pure acetone to a 1000 mL reaction flask, slowly add 14.0 g of loaded polyamine catalysts under stirring, stir evenly, and raise the temperature with a water bath, and wait for the reaction flask to When the temperature is stable at 30°C, start to pass chlorine gas into the reaction bottle for chlorination reaction. The speed of chlorine gas flow is 6.0L / h in the first 1~2h of the reaction, and the speed of 2~6h in the middle stage is 32.0L / h. In the later period of 6-18h, the rate of chlorine gas flow is 6.0L / h. After the chlorine reaction is completed, keep the temperature in the reaction bottle constant and continue to stir for 1-2 hours. After the heat preservation reaction, keep the temperature in the reaction bottle constant, add a certain amount of special solvent into the reaction bottle, and keep stirring for 1 hour. After the stirring was completed, the temperature of the obtained mixture was lowered to 10° C. with ic...
Embodiment 3
[0023] Example 3: Add 232.0 g (4.0 mol) of ultra-pure acetone to a 1000 mL reaction flask, slowly add 21.0 g of loaded polyamine catalysts under stirring, stir evenly and raise the temperature with a water bath, and wait for the reaction flask to When the temperature is stable at 30°C, start to pass chlorine gas into the reaction bottle for chlorination reaction. The speed of chlorine gas flow is 8.0L / h in the first 1~2h of the reaction, and the speed of chlorine gas flow in 2~6h in the middle stage is 28.0L / h. In the later period of 6-18 hours, the rate of chlorine gas flow is 8.0L / h. After the chlorine reaction is completed, keep the temperature in the reaction bottle constant and continue to stir for 1-2 hours. After the heat preservation reaction, keep the temperature in the reaction bottle constant, add a certain amount of special solvent into the reaction bottle, and keep stirring for 1 hour. After the stirring was completed, the temperature of the obtained mixture was ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com