A kind of efficient preparation method of new cefotaxime sulfate intermediate
A technology for cefotaxime sulfate and intermediates, which is applied in the field of preparation of cefotaxime sulfate intermediates, can solve the problems of reduced reaction yield and prolonged reaction time, and achieve the effects of high yield and short reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
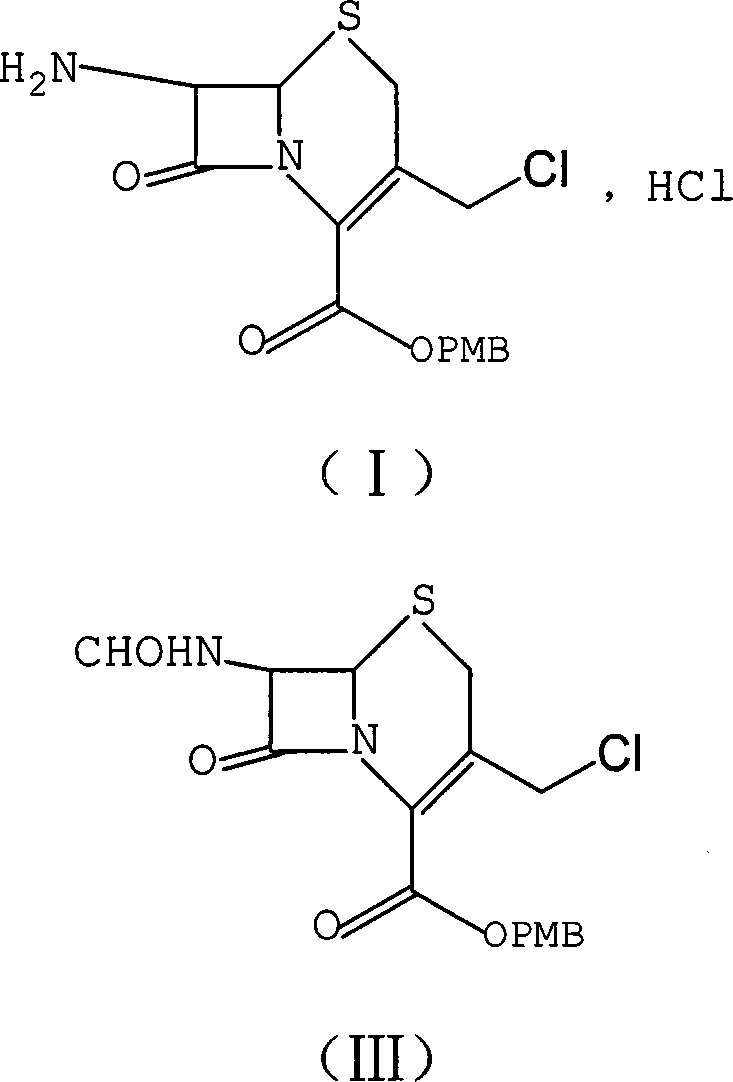
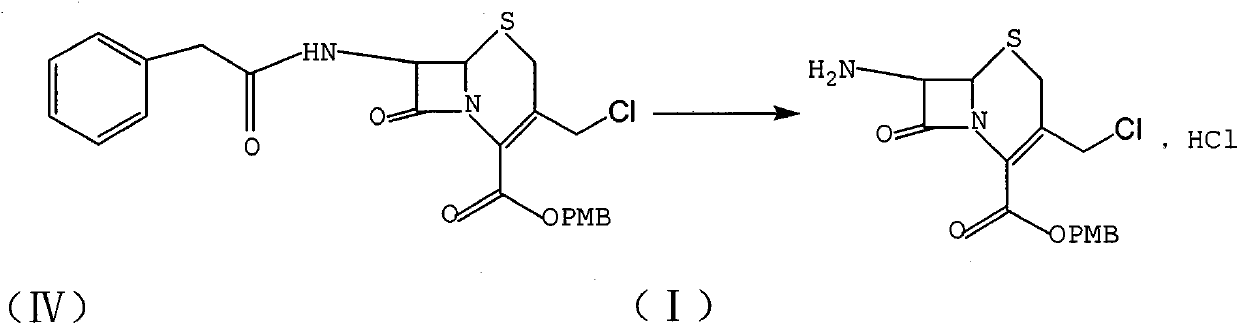
[0010] The preparation of reference example formula (I) compound
[0011]
[0012] CH 2 Cl 2 (3L), PCl 5 (374.4g) was sequentially added to a 10L reaction flask, stirred at room temperature to reduce the internal temperature to about 0°C, slowly added pyridine (200mL) dropwise, and the rate of addition was controlled so that the internal temperature did not exceed 5°C. After continuing the reaction for 1 hour, the temperature was lowered to -5°C, and GCLE (7-phenylacetamide-3-chloromethylcephemenoic acid p-methoxybenzyl ester, 584.4g) was added in batches, and the internal temperature was controlled at -5°C to 0°C. Between ℃, continue to react for 3h. The temperature was rapidly lowered to -30°C, methanol (1500 mL) was slowly added dropwise, and the reaction was continued at -30°C for 1 h. The temperature was raised to -10°C, 1500mL of water was added dropwise, and the temperature was controlled from -10 to 0°C to react for 1h. Finally, add 1500mL of water at one time,...
Embodiment 1
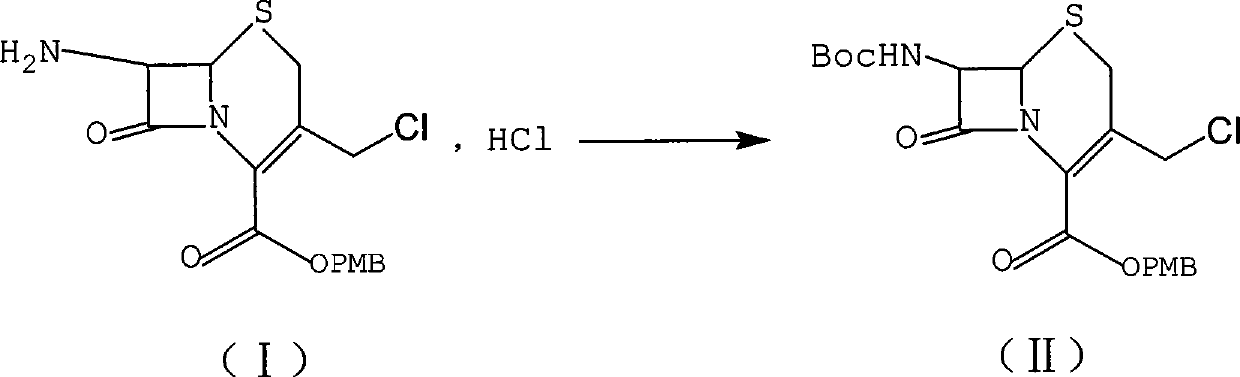
[0013] Preparation of embodiment one formula (II) compound
[0014]
[0015] Weigh the reference example product (450g) and place it in a 5L reaction flask, add 1,4-dioxane (1L), add 500mL of aqueous sodium hydroxide solution (1mol / L) gradually to adjust the pH value to 8-9, then add Benzyltriethylammonium chloride (10g), methanol 50ml, after stirring, add di-tert-butyl dicarbonate (450g), and react at 35°C for 5h. After TLC monitors that the reaction is complete, add ethyl acetate (1000mL) for extraction, separate the layers, add 150mL of ethyl acetate to the aqueous layer for extraction, separate the layers, combine the organic phases, wash with saturated brine (250mL×2), anhydrous sodium sulfate (200g ) was dried, filtered, and concentrated to obtain an oily substance, which was fully dissolved by adding 600mL of ethyl acetate and then dripping sherwood oil (1000ml) to crystallize, after stirring for 1h, suction filtered, and the filter cake was vacuum-dried at room temp...
Embodiment 2
[0017] Preparation of embodiment two formula (II) compound (referring to CN101993450A)
[0018]
[0019] Weigh the reference example product (81.0g) into a 2L reaction flask, add tetrahydrofuran (400mL), triethylamine (24.3g), cool down to 0-5°C, add di-tert-butyl dicarbonate (48.0g) dropwise, After completion of the dropwise addition, return to room temperature and stir for 8 hours to react. TLC detects that there is still a large amount of raw material formula (I) remaining. Continue to stir and react for about 12 hours. The basic reaction of the raw materials is complete. The reaction solution is concentrated to 150 mL, and 500 mL of ethyl acetate is added. Wash with 20mL of 1N hydrochloric acid and 200mL of saturated brine, dry the organic phase with anhydrous sodium sulfate, filter, concentrate the filtrate to dryness, add petroleum ether (60-90°C) to freeze and crystallize, filter, and dry to obtain 55.0g of white solid. The rate is 58.6%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com