4-Saturated cyclosubstituted aniline protein kinase inhibitor
A protein kinase inhibitor, aniline technology, applied to 4-saturated ring-substituted aniline protein kinase inhibitors, pharmaceutical use of diseases, compounds that regulate anaplastic lymphoma kinase activity, treatment or prevention of protein kinase-related diseases In the field of compounds, it can solve problems such as the incidence of adverse reactions in the digestive tract and the prolongation of QT interval
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
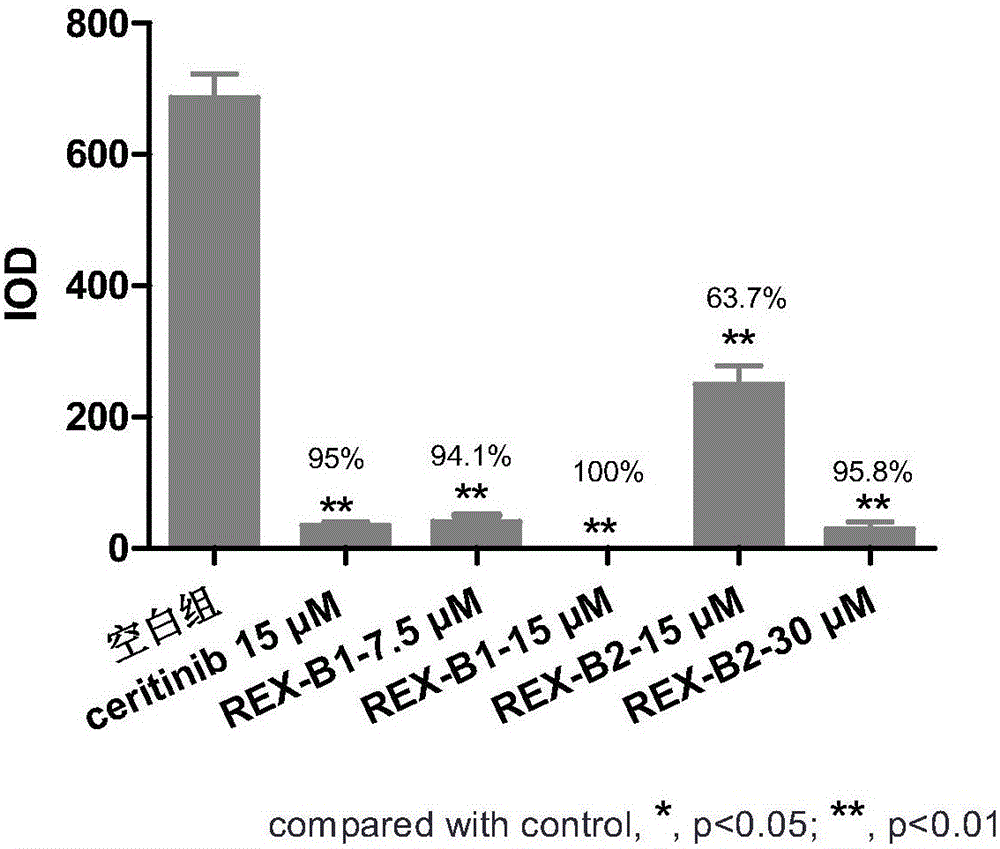
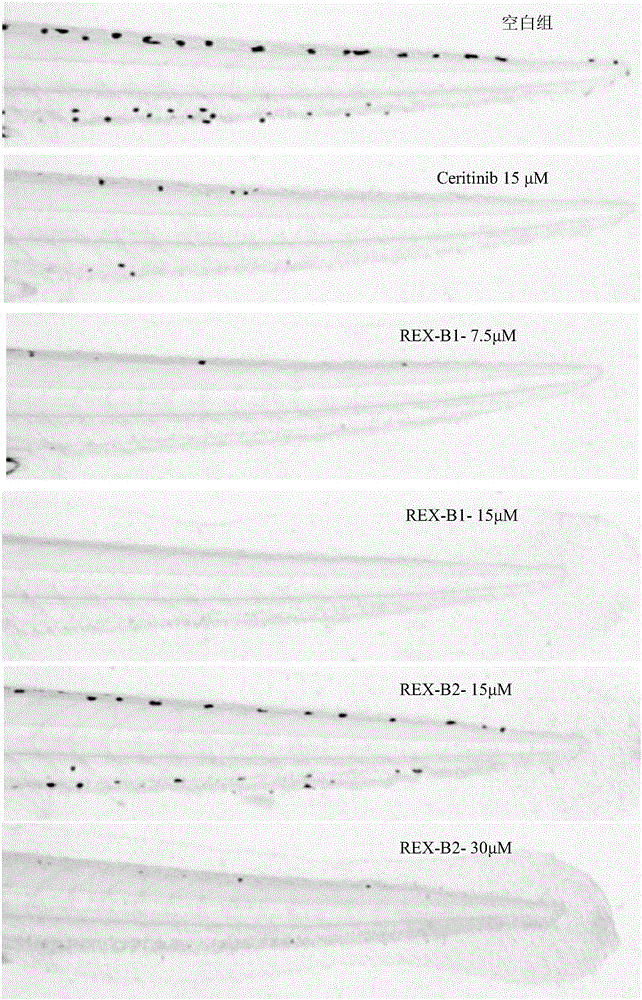
Image
Examples
Embodiment 1
[0097] Example 1 5-Chloro-N 2 -(2-isopropoxy-5-methyl-4-(1-(methylenemorpholinyl)cyclopropyl)phenyl)-N 4 Preparation of -(2-(isopropylsulfonyl)phenyl)-2,4-diaminopyrimidine [No. REX-B1]
[0098] The synthetic route is as follows:
[0099]
[0100] Synthetic Scheme 1: Synthesis of intermediate 2-isopropoxy-5-methyl-4-(1-(methylenemorpholinyl)cyclopropyl)aniline (i.e. compound 1-12)
[0101] Step 1: Preparation of intermediate 2-methyl-5-nitro-phenylacetic acid (i.e. compound 1-2)
[0102] Dissolve the raw material 2-methyl-phenylacetic acid (200.0g, 1.33mol) in dichloromethane (700mL), slowly add concentrated sulfuric acid (584mL) below 0°C, continue the reaction for 0.5 hours after the addition, and then add concentrated nitric acid dropwise (30mL), keep the temperature at -2~3°C for 16 hours; after the reaction, pour into 500mL water, extract with dichloromethane, dry and concentrate to obtain compound 1-2 (90.0g), yield: 34.6 %.
[0103] MS m / z [ESI]: 196.1 [M+1].
...
Embodiment 2
[0151] Example 2 5-Chloro-N 2 -(4-(1-((Dimethylamino)methyl)cyclopropyl)-2-isopropoxy-5-methylbenzene)-N 4 Preparation of -(2-(isopropylsulfonyl)phenyl)-2,4-diaminopyrimidine [No. REX-B2]
[0152] The synthetic route is as follows:
[0153]
[0154] As described in the synthetic route provided in this example, in the synthetic scheme 1 "synthesis of compound 1-12", in step 9, dimethylamine is used instead of morpholine in Example 1 for the reaction, and the rest of the synthetic method is the same as that of Example 1 Synthesis scheme 1, the compound 1-12 is obtained, the yield: 22.0%.
[0155] MS m / z [ESI]: 263.4 [M+1].
[0156] In Synthesis Scheme 2 "Synthesis of Compound 2-5", the raw material 2-fluoronitrotoluene (i.e. Compound 2-1) and the rest of the synthesis methods are the same as in Synthesis Scheme 2 of Example 1 to obtain Compound 2-5. Rate: 33%.
[0157] MS m / z [ESI]: 370.2 [M+1].
[0158] In the synthesis scheme 3 "synthesis of the target compound REX-B2...
Embodiment 3
[0160] Example 3 5-Chloro-N 2 -(2-isopropoxy-5-methyl-4-(1-((4-oxopiperidinyl)methyl)cyclopropyl)phenyl)-N 4 Preparation of -(2-(isopropylsulfonyl)phenyl)-2,4-diaminopyrimidine [No. REX-B3]
[0161] The synthetic route is as follows:
[0162]
[0163] As described in the synthetic route provided in this example, in Synthetic Scheme 1, since the raw materials and synthetic methods of "Step 1 to Step 7" in this example are the same as those in "Step 1 to Step 7" in Synthetic Scheme 1 of Example 1 " same, therefore can directly take the compound 1-8 that the synthetic scheme 1 of embodiment 1 makes, synthesize according to the route of step 8 to step 11, obtain intermediate compound 1-12, the preparation process is specifically as follows:
[0164] Step 8: Preparation of intermediate 1-(2-methyl-4-nitro-5-isopropoxy)cyclopropylphenylethanol (ie compound 1-9)
[0165] Compound 1-8 (500mg, 1.7mmol) was dissolved in tetrahydrofuran (50ml), and lithium aluminum hydride (128mg, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com