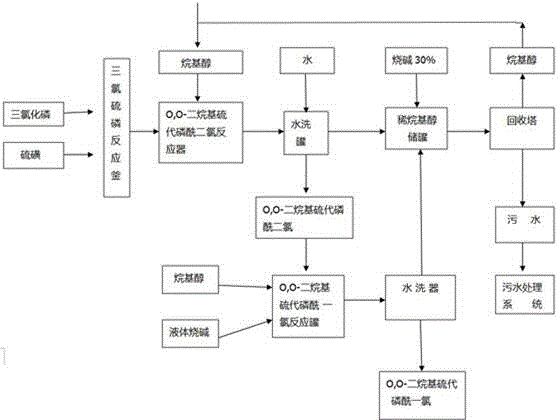
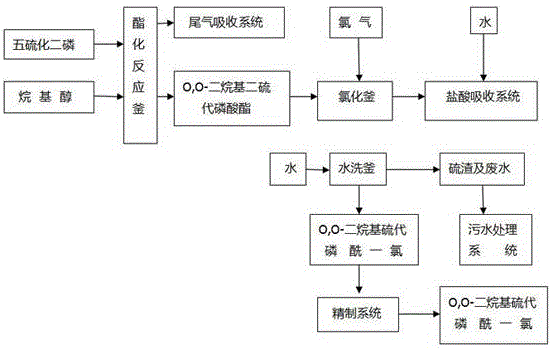
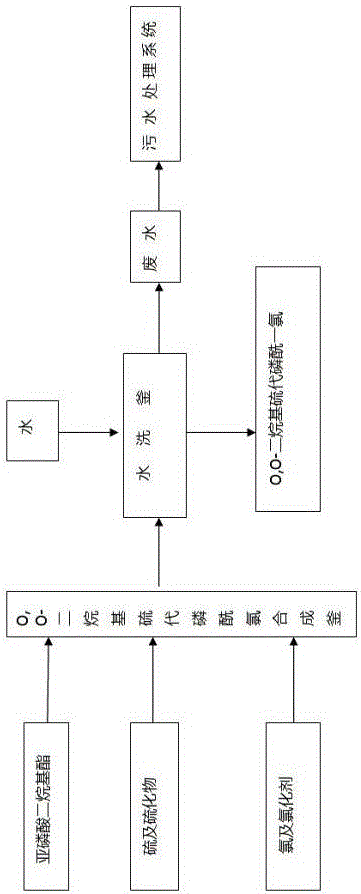
Production method for O,O-dialkyl thiophosphoryl chloride
A technology for the production of alkylphosphorylthioxate, which is applied in the field of producing O,O-dialkylphosphorylthiochloride by chemical method, can solve the problems of difficult process control, complex process, and many raw materials, and achieve high power consumption , dark color, less environmental pollution effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Put dimethyl phosphite and sulfur into the reaction kettle, raise the temperature to 70°C, keep the temperature for 2 hours, then cool down to 20°C, then slowly add the measured chlorine into the reaction kettle, after the chlorine is added, put the measured Add water into the kettle and continue to stir for one hour. After layering, the lower layer is high-quality O,O-dimethylphosphorothioate monochloride. After analysis, the product content is 99.1%, and 158.8 grams of the product are obtained. The yield 98.1% (calculated as dimethyl phosphite).
[0043] The material feeding amount in this example is as follows:
[0044] Dimethyl phosphite: 112 grams, sulfur: 33.2 grams, chlorine: 74 grams, water: 145 grams.
Embodiment 2
[0046]Put diethyl phosphite and sulfur into the reaction kettle, raise the temperature to 75°C, keep it warm for 2 hours, then cool down to 20°C, then slowly add the metered chlorine into the reaction kettle, after the chlorine is added, add the metered water Put it into the kettle, continue to stir for one hour, and after layering, the lower layer is high-quality O, O-diethylphosphorothioate monochloride. After analysis, the product content is 99.6%, and 185.6 grams of the product are obtained, with a yield of 99.0%. (calculated as diethyl phosphite).
[0047] The material feeding amount in this example is as follows:
[0048] Diethyl phosphite: 140 grams, sulfur: 33.2, chlorine: 74 grams, water 175 grams.
Embodiment 3
[0050] Put dimethyl phosphite into the reaction kettle, raise the temperature to 40°C, slowly drop sulfur chloride into the reaction kettle, keep the temperature at 40°C and finish dropping the sulfur chloride; It is passed into the material under certain conditions; after the completion of chlorine passage, a certain amount of water is added, and the layers are washed with water, and the lower layer is O, O-dimethylphosphorylthiochloride. After analysis, the product content is 99.5%, and the product is obtained 316.2 grams, yield 98.6% (calculated as dimethyl phosphite).
[0051] The material feeding amount in this example is as follows:
[0052] Dimethyl phosphite: 224 grams, sulfur chloride: 138 grams, chlorine: 72 grams, water: 365 grams.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com