Procalcitonin immunofluorescence quantitation test strip and preparation method thereof
A procalcitonin, immunofluorescence technology, applied in the field of immunological detection, can solve the problem of high requirements for specialization of venues, equipment and personnel, and achieve the effects of good storage stability, high accuracy and good precision
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] The preparation process of procalcitonin immunofluorescence quantitative test strip is as follows:
[0025] (1) The preparation of preferred stock solution: the mass concentration of PB is 20mM, the percentage concentration of BSA is 1.8%, the percentage concentration of Tween-80 is 0.5%, the percentage concentration of glucose is 0.5%, the percentage concentration of glycine The percentage concentration of PEG4000 is 1%, the percentage concentration of PEG20000 is 1.5%, and the percentage concentration of Proclin300 is 0.03%. After mixing according to the above formula, filter and sterilize to prepare the storage solution;
[0026] (2) Preparation of the sample pad: soak the glass fiber membrane with the sample pad treatment solution for 10 minutes, place in a drying room at 37°C and 30% humidity, and dry for 3 hours to prepare the sample pad for use;
[0027] (3) Preparation of the bonding pad: Soak the glass cellulose membrane with the bonding pad treatment solution ...
Embodiment approach
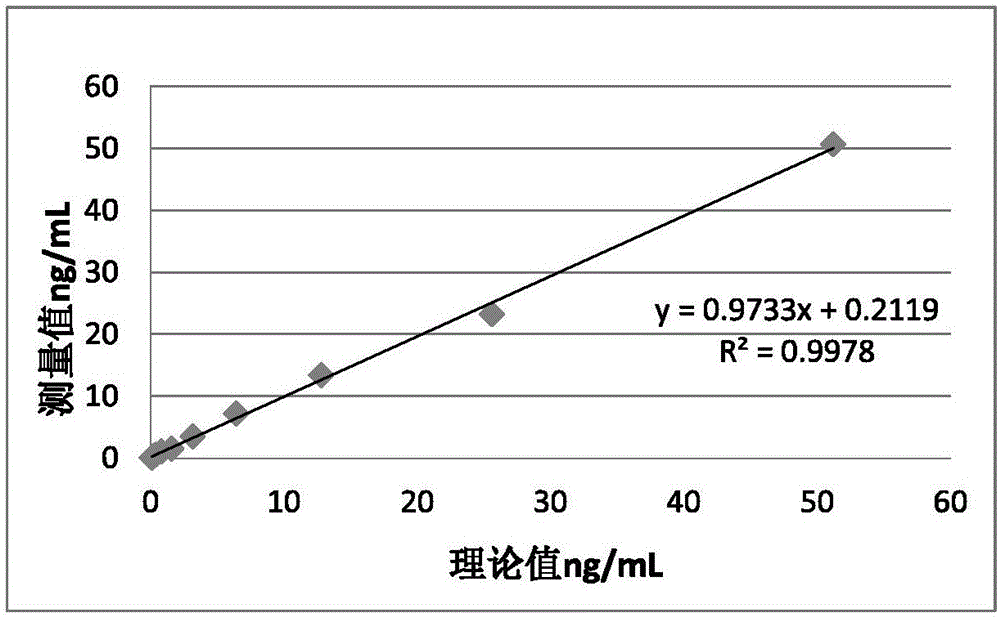
[0041] The procalcitonin immunofluorescence quantitative test strip prepared in Example 1 is used to establish a standard curve and detect it. The implementation method is as follows:
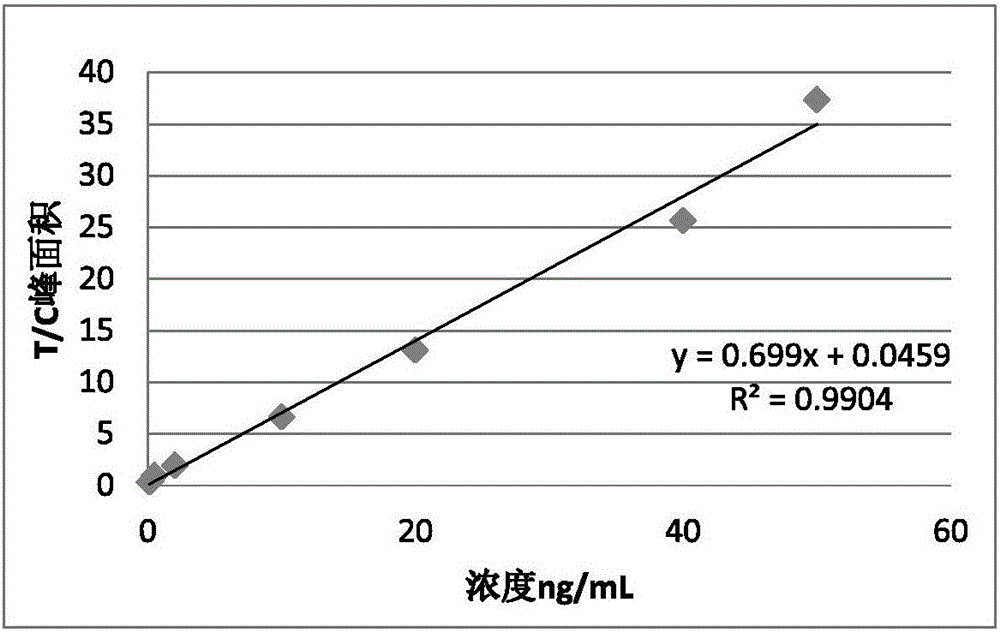
[0042](1) Establish a standard curve: mix and dilute to 0.1ng / mL, 0.25ng / mL, 0.5ng / mL, 2ng / mL, 10ng / mL with Roche procalcitonin concentration of 54ng / mL and 0.1ng / mL standard , 20ng / mL, 40ng / mL, 50ng / mL eight concentrations. Add 80 μL of prepared test strips to immunofluorescence analysis for detection, and repeat 3 times for each concentration to obtain the average value. Take the ratio of the T peak area of the detection line and the C peak area of the quality control line as the ordinate, and the theoretical value of the standard product as the abscissa, to obtain the linear regression equation;
[0043] standard curve as figure 1 As shown, the linear relationship y=0.699x+0.0459, R 2 =0.9904, this equation can be used to calculate the content of procalcitonin in the sample, to achiev...
Embodiment 3
[0047] Prepare the control storage solution, the specific formula is: the mass concentration of PB is 50mM, the percentage concentration of BSA is 1%, the percentage concentration of Tween-80 is 1%, the percentage concentration of glucose is 1%, and the percentage concentration of glycine 0.5%, the percentage concentration of PEG4000 is 2%, and the percentage concentration of Proclin300 is 0.3%.
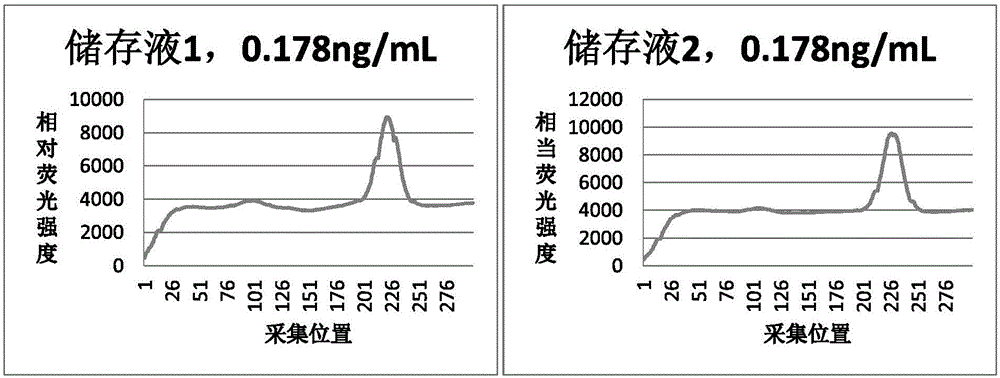
[0048] The preferred storage solution prepared in Example 1 (hereinafter referred to as storage solution 2) is compared with the reference storage solution (hereinafter referred to as storage solution 1) for stabilizing performance and precision, and the implementation method and results are as follows:
[0049] Using storage solution 1 and storage solution 2, the procalcitonin (PCT) primary antibody-fluorescent microspheres labeled with the same process were sprayed and dried, assembled into 40 test strips, put in aluminum foil bags, and sealed with a desiccant. One bag was stored i...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com