O-alpha-methyl mandelate-N-trimethyl chitosan quaternary ammonium salt as well as preparation method and application thereof
A technology of trimethyl chitosan and methyl mandelate, applied in the direction of antibacterial drugs, etc., can solve problems such as insolubility in water, and achieve enhanced antibacterial activity and hydrophilicity, good antibacterial properties, and expanded application scope. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
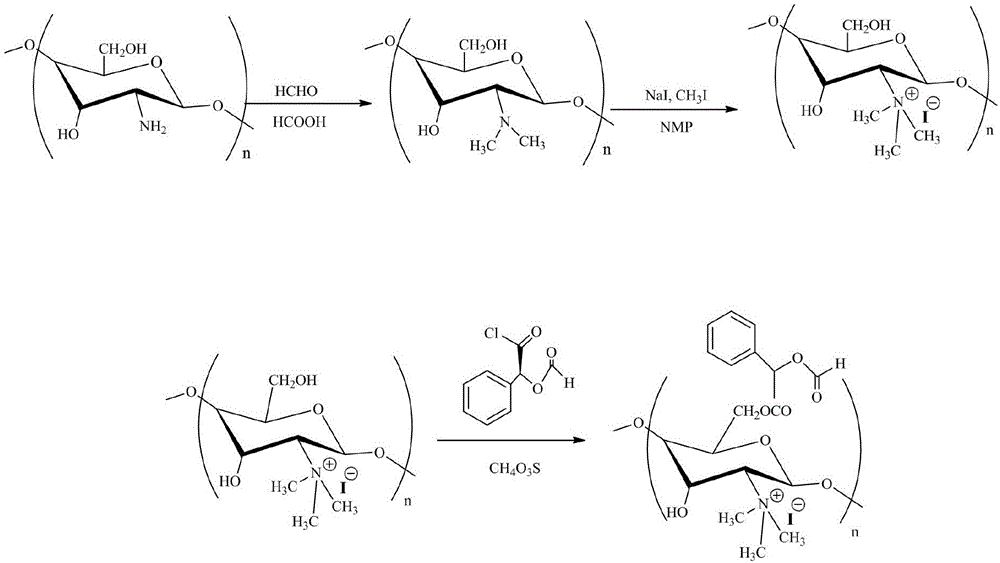
[0029] A kind of O-alpha-methyl mandelic acid ester-N-trimethyl chitosan quaternary ammonium salt, its preparation method is as follows:
[0030] (1) Dissolve 2g of chitosan in 100mL of 15% formic acid solution, stir and heat up to 50 oC , add 6mL of formaldehyde solution, mix evenly and transfer to a microwave normal pressure reactor, adjust the microwave power to 200W, temperature 50 oC React for 40 minutes; adjust pH to alkaline with 10% NaOH solution, filter with suction, wash with water until neutral, add to 50mL N-methyl-2-pyrrolidone after drying, add 6mL methyl iodide, 50 oC React for 120 hours; add twice the volume of V (ether) / V (ethanol) = 1:1 mixed solvent for precipitation, use deionized water to dialyze and freeze-dry to obtain N,N,N-trimethyl chitosan.
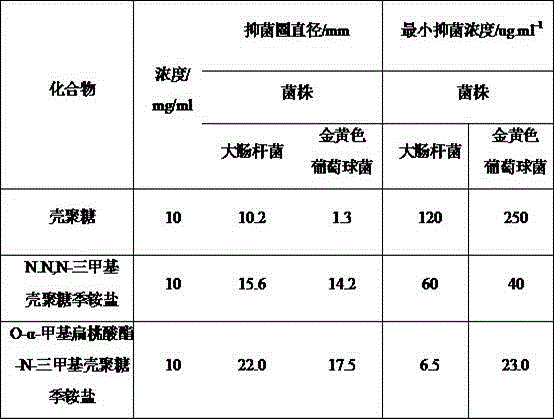
[0031] The sample was detected by X-ray photoelectron spectroscopy (XPS), and the N-position quaternization substitution degree of N,N,N-trimethyl chitosan was measured to be 76%.
[0032] (2) Weigh 1g of N,N,...
Embodiment 2
[0035] A kind of O-alpha-methyl mandelic acid ester-N-trimethyl chitosan quaternary ammonium salt, its preparation method is as follows:
[0036] (1) Dissolve 2g of chitosan in 100mL of 15% formic acid solution, stir and heat up to 50 oC , add 6mL of formaldehyde solution, mix evenly and transfer to microwave normal pressure reactor, adjust microwave power to 400W, temperature 60 oCReact for 30 minutes; use 10% NaOH solution to adjust the pH to alkaline, filter with suction, wash with water until neutral, add to 50mL N-methyl-2-pyrrolidone after drying, add 12mL methyl iodide, 60 oC React for 80 minutes; add twice the volume of V (ether) / V (ethanol) = 1:1 mixed solvent for precipitation, use deionized water to dialyze and freeze-dry to obtain N,N,N-trimethyl chitosan.
[0037] The sample was detected by X-ray photoelectron spectroscopy (XPS), and the N-position quaternization substitution degree of N,N,N-trimethyl chitosan was measured to be 79%.
[0038] (2) Weigh 1g of N,N...
Embodiment 3
[0041] A kind of O-alpha-methyl mandelic acid ester-N-trimethyl chitosan quaternary ammonium salt, its preparation method is as follows:
[0042] ((1) Dissolve 2g of chitosan in 100mL of 15% formic acid solution, stir and heat up to 50 oC , add 6mL of formaldehyde solution, mix evenly and transfer to microwave normal pressure reactor, adjust microwave power to 600W, temperature 70 oC React for 20 minutes; use 10% NaOH solution to adjust the pH to alkaline, filter with suction, wash with water until neutral, add to 50mL N-methyl-2-pyrrolidone after drying, add 18mL methyl iodide, 70 oC React for 40 minutes; add twice the volume of V (ether) / V (ethanol) = 1:1 mixed solvent for precipitation, use deionized water to dialyze and freeze-dry to obtain N,N,N-trimethyl chitosan.
[0043] The sample was detected by X-ray photoelectron spectroscopy (XPS), and the N-position quaternization substitution degree of N,N,N-trimethyl chitosan was measured to be 85%.
[0044] (2) Weigh 1g of N...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com