Preparation of multiple-target fusion protein resistant to tumor invasion and metastasis and application of multiple-target fusion protein
A fusion protein and multi-target technology, which is applied in the field of bioengineering pharmaceutical proteins, can solve the problems of anti-tumor effects of NGR peptides and sTRAIL coupling that have not yet been seen, and achieve enhanced anti-tumor effects, significant anti-proliferation effects, and inhibitory effects. The effect of internal and external transfer
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] The following examples only help those skilled in the art to better understand the present invention, but do not limit the present invention in any way. "Example 1" Construction of recombinant expression vectors pHBM-rgd-trail-ngr, pHBM-rgd-trail and pHBM-trail-ngr
[0050] Recombinant plasmids pHBM-rgd-trail-ngr and pHBM-trail-ngr were constructed using pHBM expression vector using restriction enzyme digestion, PCR, and ligation molecular biology techniques.
[0051] The complete gene sequence of the fusion protein RGD-connecting peptide (G4S)-TRAIL-connecting peptide (G4S)-NGR was optimized and synthesized by GenScript according to the preferred codons of Pichia pastoris. The gene sequences of the fusion proteins RGD-TRAIL and TRAIL-NGR passed The design primers are constructed using molecular biology technology, and the primers are constructed by Invitrogen TM Synthesized by the company, the expression vector is pHBM (CHKD full-text database of doctoral and master's thes...
Embodiment 2
[0064] "Example 2" Expression and purification of TRAIL-related fusion protein in Pichia pastoris
[0065] The constructed expression plasmids pHBM-rgd-trail, pHBM-trail-ngr, and pHBM-rgd-trail-ngr were transformed into DH5α competent cells. After double enzyme digestion and sequencing, the plasmid was extracted and linearized with Sal I. figure 1 ), gel to recover fragments with large molecular weight, electrotransform Pichia pastoris GS115 competent cells, spread on MD plates, pick single colonies for colony PCR identification, pick His + The colony is induced to express, and a relatively high expression strain is selected as the expression strain for large-scale fermentation. The strain was named GS115-RTN and was sent to the General Microbiology Center of China Microbial Culture Collection and Management Committee on September 19, 2016, and its number is CGMCC No.13016. First inoculate the fusion protein expression strain on BMGY medium (1% yeast extract, 2% peptone, 1.34% YN...
Embodiment 3
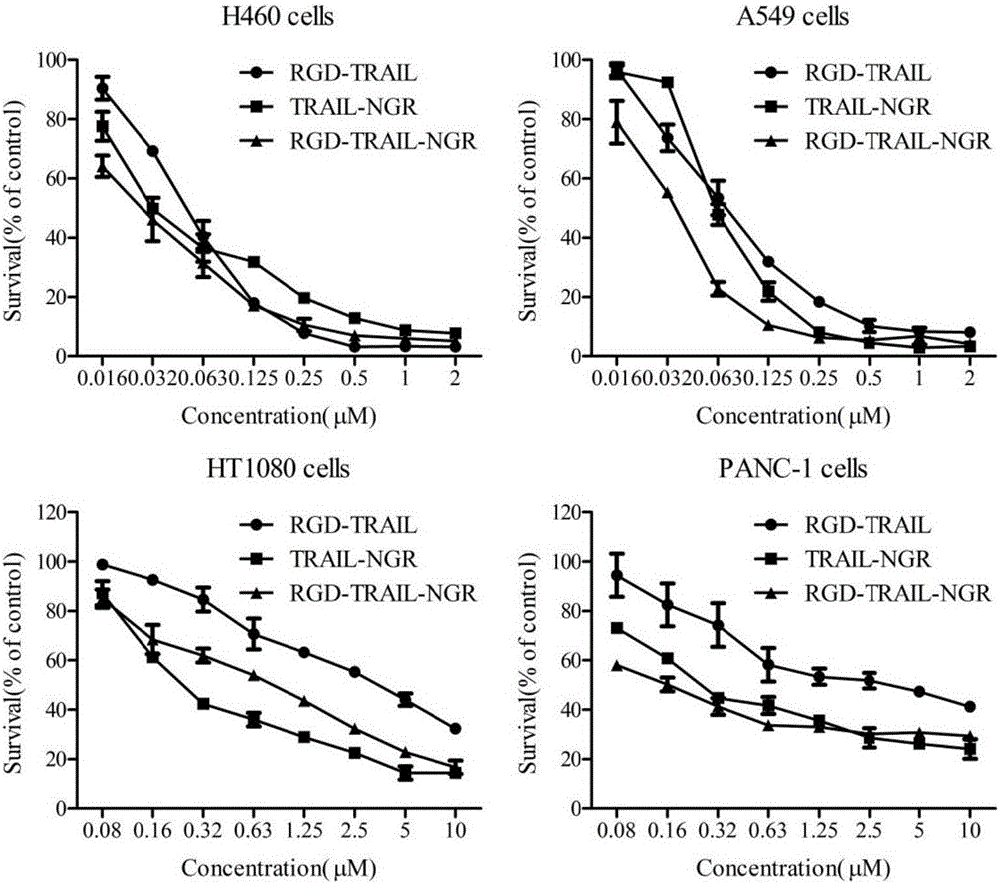
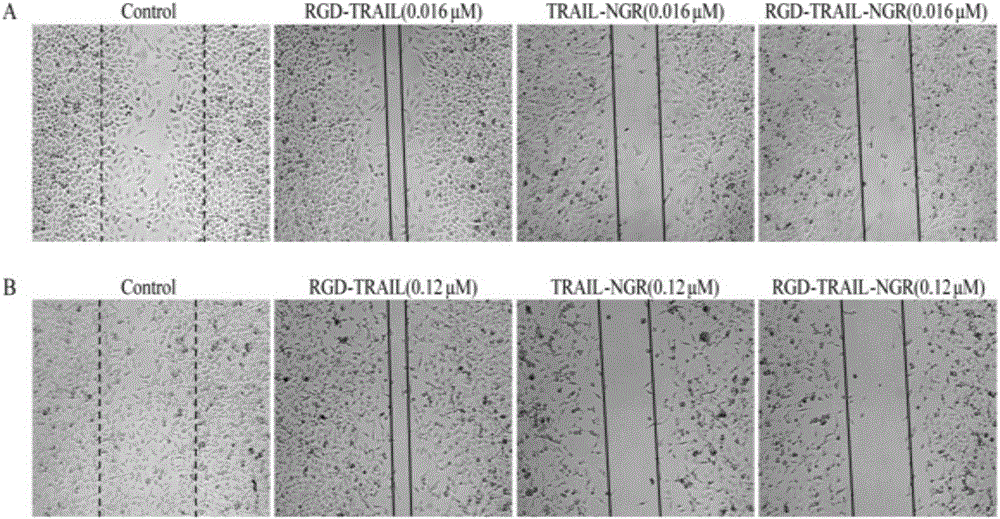
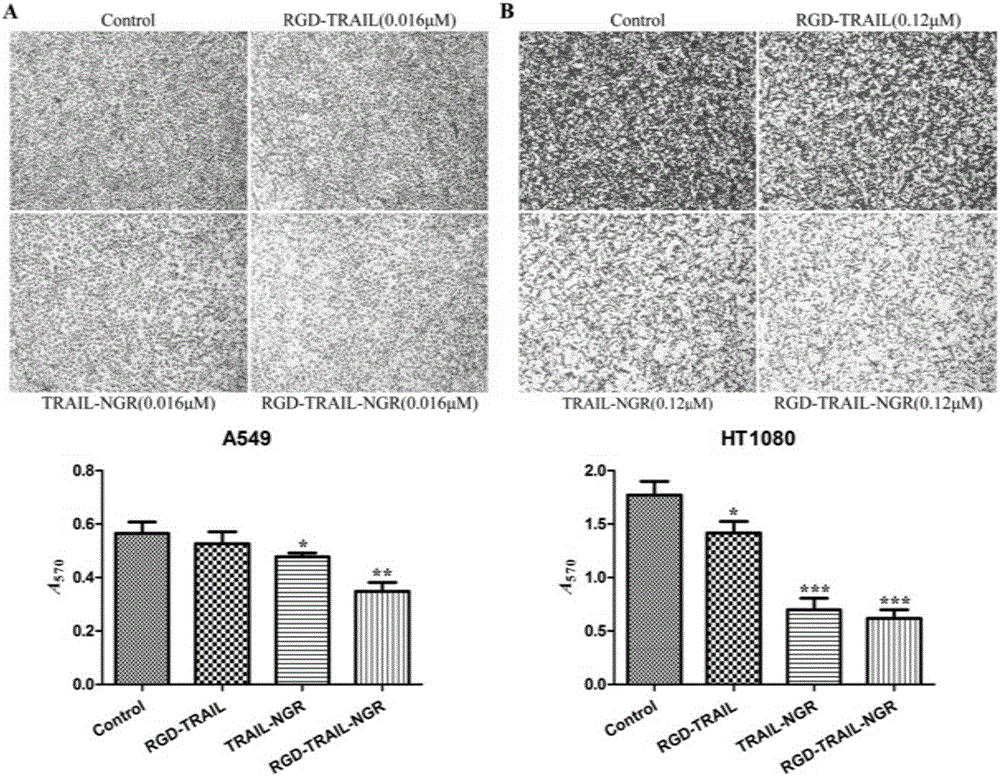
[0066] "Example 3" Analysis of the affinity activity between the fusion protein and tumor cells
[0067] 1) Western blot detection of α on the surface of different tumor cells ν , CD13, DR4 and DR5 expression levels
[0068] Tumor cells HEK293, 3T3, A549, H460, PANC-1, BxPC-3, MIA, HCT-15 and HT1080 are all passaged and preserved in this room. The tumor cells in the logarithmic growth phase were rinsed three times with pre-cooled PBS, and an appropriate amount of cell lysate (50mmol / L Tris-Hcl, 150mmol / L Nacl, 0.2% SDS, 2% NP-40, 0.5% Sodium deoxycholate, pH 8.0. Add 1% PMSF just before use, and lyse on ice for 30 minutes. Centrifuge at 4°C and 12000 rpm for 20 minutes, and transfer the supernatant to a new 1.5 ml EP tube. Use the BCA kit to quantify the protein of various cell lysis samples. Each cell lysis sample is prepared with the same total protein amount, mixed with an appropriate amount of 5× loading buffer, denatured in a boiling water bath for 10 minutes, and loaded aft...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com