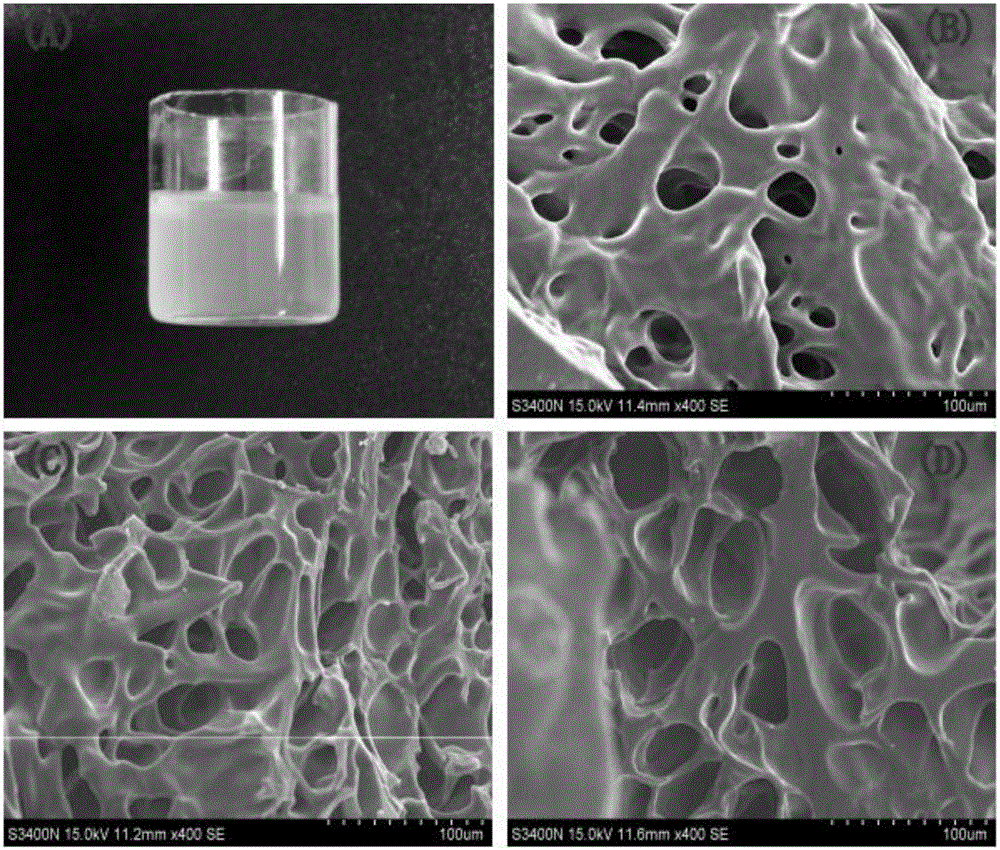
Preparation and application of 1,8-diamino-p-methane hyperbranched polyether amine and its hybrid hydrogel
A hyperbranched polyetheramine, hydrogel technology, applied in the directions of alkali metal oxides/hydroxides, inorganic chemistry, water pollutants, etc. The post-processing process is simple and the effect of improving the degree of branching
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
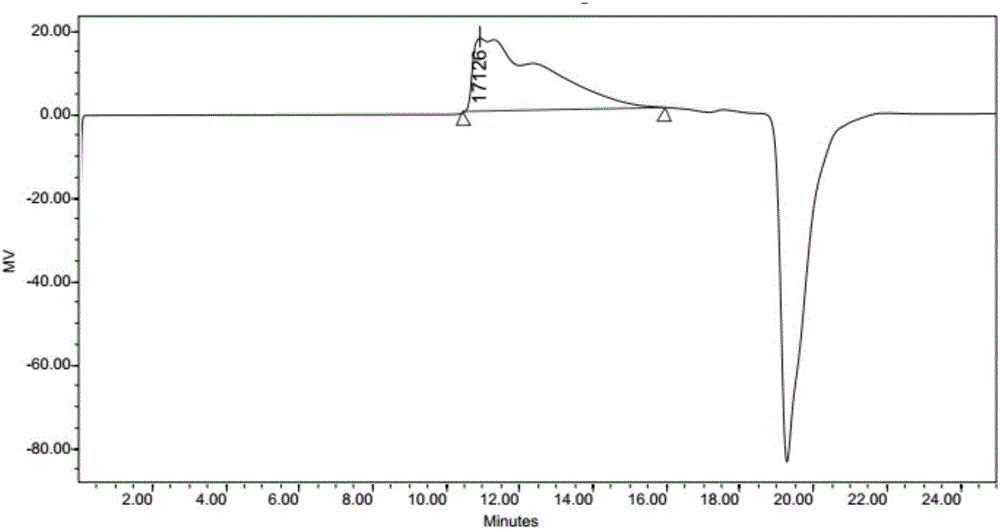
[0046] Weigh 3.4g (0.02mol) of 1,8-p-mendiamine, add 8.5mL of absolute ethanol, stir magnetically, and pass nitrogen gas for half an hour. Weigh 20g (0.04mmol) of polyethylene glycol diglycidyl ether (Mn=500) and dissolve it in 50mL of ethanol, slowly drop it into the reaction solution, after the dropwise addition, stir for 24h, then raise the temperature to 85°C and react for 24h to obtain The reaction solution was distilled under reduced pressure to remove ethanol under the condition of vacuum degree of 0.086MPa, then settled with petroleum ether for many times, discarded the upper layer liquid, and the lower layer paste was vacuum-dried under the condition of vacuum degree of 0.09MPa for 6h to obtain the overrun Polyetheramines. as attached figure 2 As shown, the product is measured by GPC, and the GPC retention time RT of the obtained hyperbranched polyetheramine is 10.9min, its number average molecular mass Mn is 3.8K, and the weight average molecular mass Mn is 3.8K. ...
Embodiment 2
[0049] Weigh 3.4g (0.02mol) of 1,8-p-mendiamine, add 8.5mL of absolute ethanol, stir magnetically, and pass nitrogen gas for half an hour. Weigh 38.4g (0.06mol) of polypropylene alcohol diglycidyl ether (Mn=640) and dissolve it in 64mL of ethanol, and slowly drop it into the reaction solution. After the dropwise addition, stir for 24h, then raise the temperature to 80°C for 30h, and the obtained reaction The liquid was distilled under reduced pressure to remove ethanol under the condition of vacuum degree of 0.09MPa, and then settled with n-hexane several times, the upper layer liquid was discarded, and the lower layer paste was vacuum-dried under the condition of vacuum degree of 0.09MPa for 6h to obtain hyperbranched Polyetheramine.
Embodiment 3
[0051] Weigh 1.7g (0.01mol) of 1,8-p-mentediamine, add 10mL of absolute ethanol, stir magnetically, and pass nitrogen gas for half an hour. Weigh 6.4g (0.01mol) of polypropylene alcohol diglycidyl ether (Mn=640) and 5g (0.01mol) of polyethylene glycol diglycidyl ether (Mn=500) and dissolve them in 60mL of ethanol, slowly drop them into the reaction solution, After the dropwise addition, stir for 24 hours, then raise the temperature to 85°C and react for 24 hours. The obtained reaction liquid is distilled under reduced pressure to remove ethanol under the condition of vacuum degree of 0.09 MPa, and then settle with n-hexane for many times. The upper layer is discarded, and the lower layer is paste-like The product was vacuum-dried for 4 hours under the condition of vacuum degree of 0.09MPa to obtain hyperbranched polyetheramine.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Adsorption capacity | aaaaa | aaaaa |
| Compressive strength | aaaaa | aaaaa |
| Number average molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com