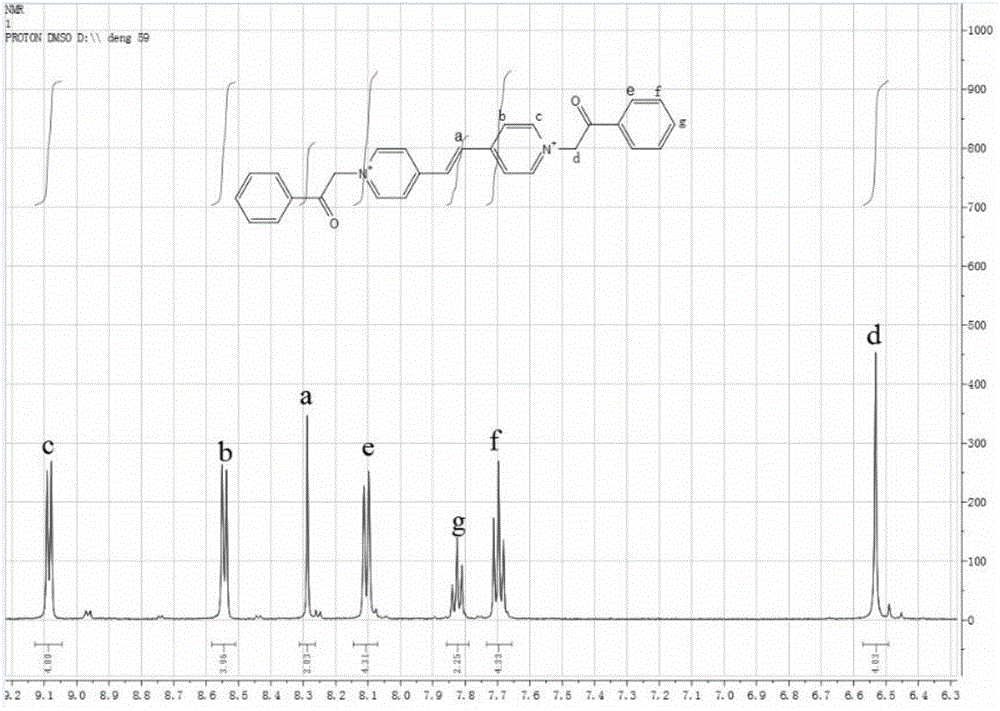
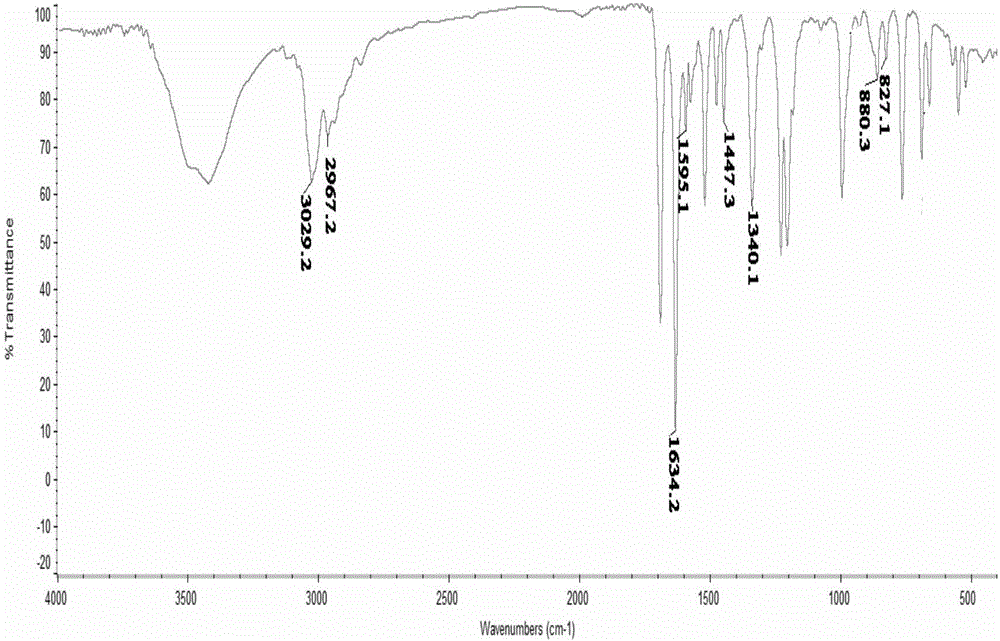
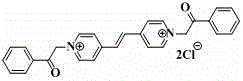
High sensitivity color-changing acetophenone substituent viologen compound and synthesis method thereof
A technology with high sensitivity and synthesis method, applied in the field of viologen compounds and their synthesis, can solve the problems of non-persistence, limited application, unstable color, etc., and achieve the effects of simple operation, improved response sensitivity, and low solvent toxicity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0017] Embodiment: a kind of synthetic method of the viologen compound of the acetophenone substituent of a kind of above-mentioned highly sensitive discoloration, this method has the following steps:
[0018] (1). First, take 1.56g 10mmol of 1,2-bis(4-pyridine)ethylene and 3.87g 25mmol of α-chloroacetophenone in a molar ratio of 1:2.5 and mix them in the reaction flask, dissolve them to 18~ In 20mL of anhydrous N, N-dimethylformamide (DMF); reflux reaction at 110-120°C for 24-25h, after the reaction, a light yellow precipitate is obtained, and its synthetic reaction formula is:
[0019]
[0020] (2). Cool the light yellow precipitate after the above reaction to room temperature, centrifuge to obtain the precipitate, wash with anhydrous N, N-dimethylformamide (DMF), high-purity water and acetone for 4-5 times each. Once, the washing liquid turns from brown to colorless; after centrifugation and vacuum drying for 8-9 hours, 3.45 g of the viologen compound with a concentratio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com