Quinazolinone compounds and preparation method thereof
A compound and quinazolone technology, applied in the field of quinazolone compounds and preparation thereof, can solve problems such as no relevant reports on the synthesis method of quinazolone compounds, and achieve the effects of easy large-scale production and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0031] The preparation method of described a kind of quinazolones compound, comprises the following steps:
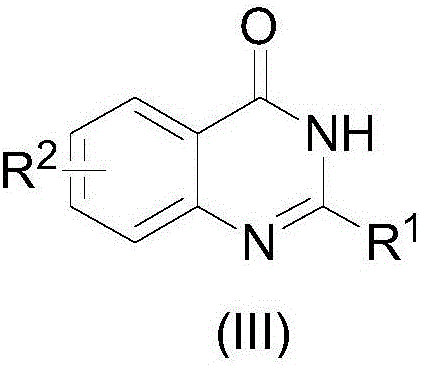
[0032] a) Alcohols with structure (I) and o-nitrobenzonitriles with structure (II) on Au / TiO 2 Under the catalysis, by stirring and heating in a solvent, the quinazolones compound of the present invention with structure (III) can be obtained:
[0033]
[0034] Among them, R 1 Is aryl, substituted aryl; R 2 is fluorine, chlorine, methyl or methoxy.
[0035] The R 1 is aryl, and said aryl is phenyl, naphthyl or thienyl;
[0036] The R 1 is a substituted aryl group, and the substituted aryl group is 4-chlorophenyl, 4-fluorophenyl, 3-fluorophenyl, 2-fluorophenyl, 4-methylphenyl, 3-methylphenyl, 3,4-dimethoxyphenyl, 4-methoxyphenyl, 3-methoxyphenyl, 2-methoxyphenyl;
[0037] The R 2 is fluorine, chlorine, methyl or methoxy;
[0038] The catalyst is Au / TiO 2 ;
[0039] The molar ratio of the o-nitrobenzonitrile compound and alcohol is 1:1-1:3;
[0040] The o-ni...
Embodiment 1
[0045] In a clean and dry 20 ml Schlenk reaction tube, sequentially add 1% Au / TiO 2 49 mg, 37 mg of o-nitrobenzonitrile, 81 mg of benzyl alcohol, and 2 ml of water were used as solvents, and a reflux tube was installed to react at 130° C. for 20 hours under nitrogen protection. After the reaction is finished, the recovered catalyst can be directly recycled next time by filtering, and the filtrate is directly spin-dried and dissolved with a small amount of petroleum ether and ethyl acetate (volume ratio of 3:1), separated by a short silica gel column, and obtained 49.9 mg of white solid, 90% yield.
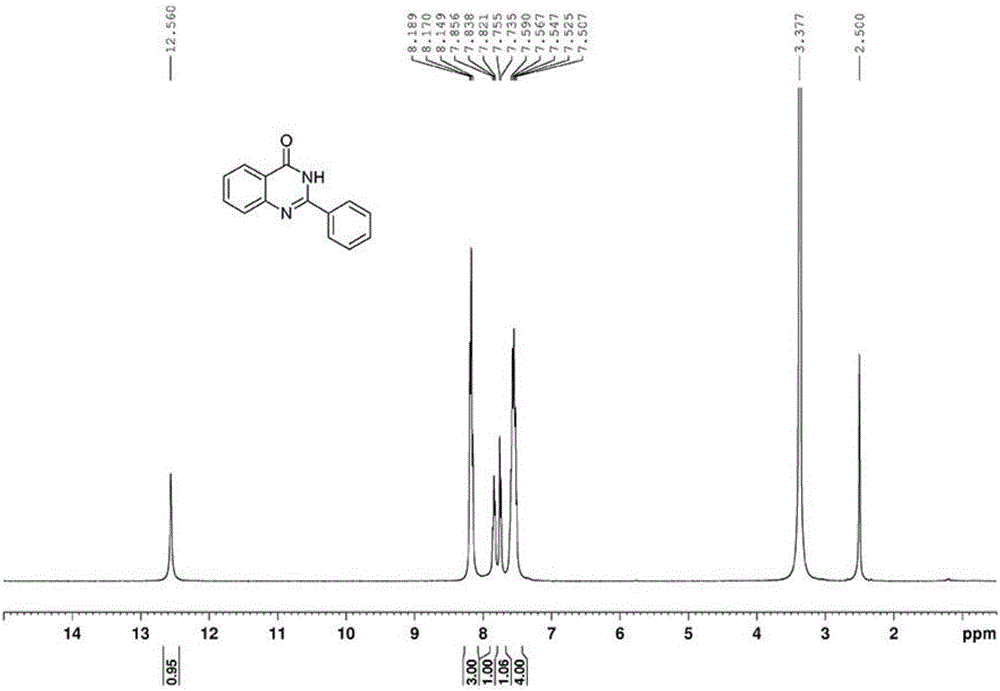
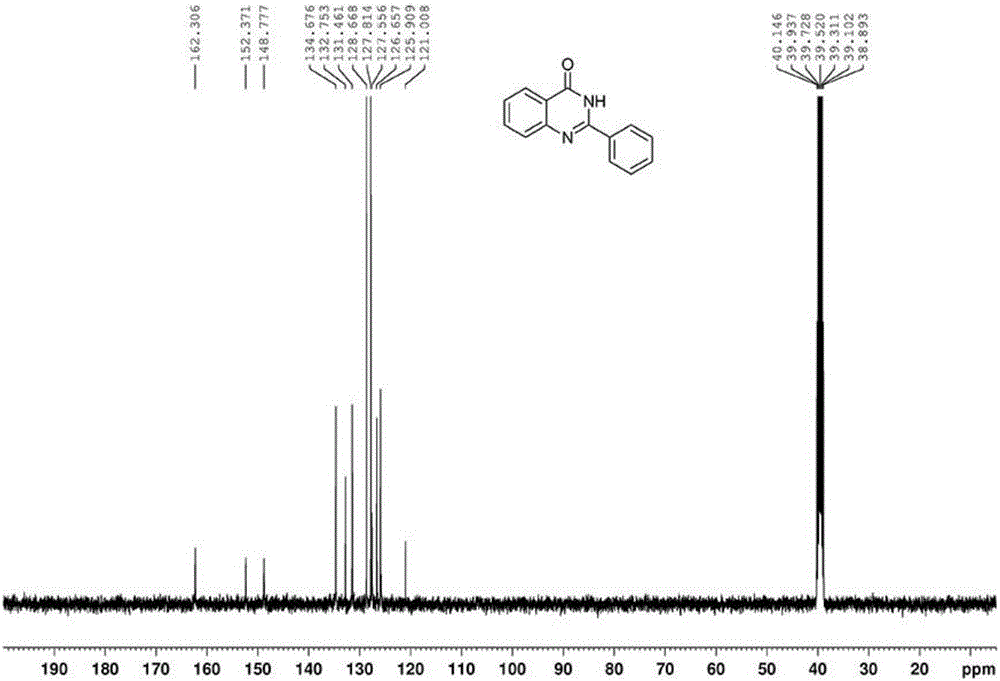
[0046] The proton nuclear magnetic resonance spectrum of the product prepared in this embodiment is as follows: figure 1 As shown, the carbon NMR spectrum is as figure 2 shown. It can be confirmed from the spectrum that the obtained product is 2-phenylquinazolone.
Embodiment 2
[0048] In a clean and dry 20 ml Schlenk reaction tube, sequentially add 1% Au / TiO 2 49 mg, 37 mg of o-nitrobenzonitrile, 70 mg of benzyl alcohol, and 2 ml of toluene were used as solvents, and a reflux tube was installed to react at 130° C. for 24 hours under nitrogen protection. After the reaction is finished, the recovered catalyst can be directly recycled next time by filtering, and the filtrate is directly spin-dried and dissolved with a small amount of petroleum ether and ethyl acetate (volume ratio of 3:1), separated by a short silica gel column, and obtained 44 mg of white solid, 80% yield.
[0049] The proton nuclear magnetic resonance spectrum of the product prepared in this embodiment is as follows: figure 1 As shown, the carbon NMR spectrum is as figure 2 shown. It can be confirmed from the spectrum that the obtained product is 2-phenylquinazolone.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com