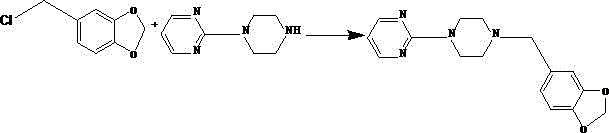
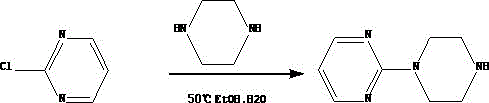Synthetic method of piribedil
A technology for piribedil and piperazine, applied in the field of synthesizing piribedil, can solve the problems of complicated operation, unfavorable industrialization, difficult industrialization, etc., and achieve the effects of simplifying synthesis steps, improving utilization rate, and reducing the generation of three wastes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0025] Reaction example 1 (Chinese patent 1884280)
[0026] 1 Preparation of piperonyl piperazine
[0027] Add 39.2g piperonal, 34.4g piperazine, 400ml methyl tert-butyl ether and 7g10% palladium charcoal catalyst in the high-pressure hydrogenation reactor, replace the air with hydrogen for 3 times after nitrogen replacement air 3 times, under 10kg hydrogen pressure, Heat the reaction at 50~60°C for 15~20 hours, lower the temperature, filter the catalyst, cool the filtrate to 13~18°C, filter the precipitated piperazine, add 150ml of water to the filtrate, keep it warm at 13~18°C, adjust the pH to 7.9~ with 7N hydrochloric acid 8.0. Separate the liquid, extract the water phase with toluene 3 times, combine the water phases, adjust the pH to 12 with 20 g of sodium hydroxide under ice bath, extract again with toluene 3 times, combine the organic phases, dry and filter with anhydrous magnesium sulfate, and concentrate to obtain 45 g Pale yellow viscous substance, yield 78%.
[...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com