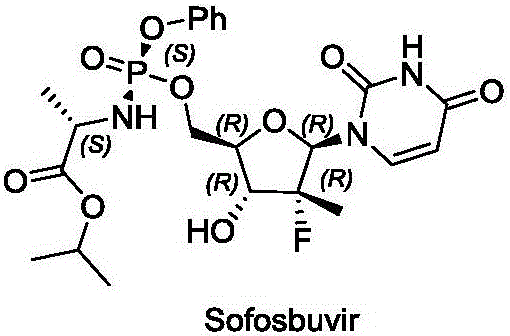
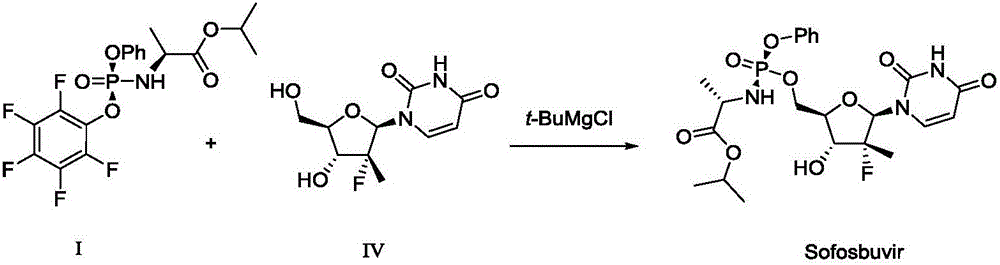
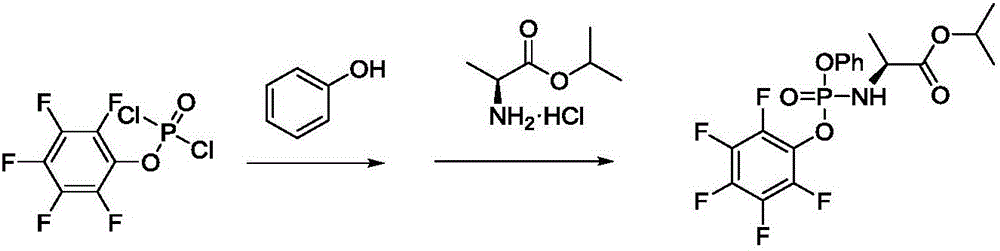
Method for preparing sofosbuvir intermediate
A technology for intermediates and crude products, applied in the field of medicinal chemistry, can solve the problems of harsh reaction conditions, high equipment requirements, low yield, etc., and achieve the effect of simple and convenient post-processing and mild reaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Part 1: Preparation of N-dichlorophosphoryl-L-alanine isopropyl ester (II)
[0040]
[0041] Step a), L-alanine isopropyl ester hydrochloride (8.4g, 50mmol), add 100ml of diethyl ether, cool down in an ice bath, control the temperature at 0°C, slowly add phosphorus oxychloride (7.7g, 50mmol), dropwise After the addition, triethylamine (10.1g, 100mmol) was slowly added dropwise. After the dropwise addition, the insulation reaction was carried out for 1 hour. After the reaction was completed, it was gradually raised to room temperature, and the insolubles were removed by suction filtration. The filtrate was concentrated under reduced pressure to obtain a yellow solid ( Compound shown in formula II), this crude product is directly used in the next step reaction without refining;
[0042] Part II: Preparation of N-[(S)-(2,3,4,5,6-pentafluorophenoxy)]phenoxyphosphoryl-L-alanine isopropyl ester
[0043]
[0044]Step b), the crude product (12.4g, 50mmol) of the compound...
Embodiment 2
[0047] Part 1: Preparation of N-dichlorophosphoryl-L-alanine isopropyl ester (II)
[0048]
[0049] Step a), L-alanine isopropyl ester hydrochloride (8.4g, 50mmol), add 100ml of tetrahydrofuran, cool down in an ice bath, control the temperature at 0°C, slowly add phosphorus oxychloride (7.7g, 50mmol), dropwise After the addition, triethylamine (10.1g, 100mmol) was slowly added dropwise. After the dropwise addition, the insulation reaction was carried out for 1 hour. After the reaction was completed, it was gradually raised to room temperature, and the insolubles were removed by suction filtration. The filtrate was concentrated under reduced pressure to obtain a yellow solid ( Compound shown in formula II), this crude product is directly used in the next step reaction without refining;
[0050] Part II: Preparation of N-[(S)-(2,3,4,5,6-pentafluorophenoxy)]phenoxyphosphoryl-L-alanine isopropyl ester
[0051]
[0052] Step b), the crude product of the compound represented ...
Embodiment 3
[0055] Part 1: Preparation of N-dichlorophosphoryl-L-alanine isopropyl ester (II)
[0056]
[0057] Step a), L-alanine isopropyl ester hydrochloride (10.0g, 50mmol), add 100ml of tetrahydrofuran, cool down in an ice bath, control the temperature at 0°C, slowly add phosphorus oxychloride (7.7g, 50mmol), dropwise After the addition, N,N-diisopropylethylenediamine (14.4g, 100mmol) was slowly added dropwise. After the dropwise addition was completed, the reaction was incubated for 1 hour. Concentration under reduced pressure gave a yellow solid (compound shown in formula II), and the crude product was directly used in the next step without purification;
[0058] Part II: Preparation of N-[(S)-(2,3,4,5,6-pentafluorophenoxy)]phenoxyphosphoryl-L-alanine isopropyl ester
[0059]
[0060] Step b), the crude product of the compound represented by formula II in step a) (12.4g, 50mmol), add 50ml of dichloromethane, cool down in an ice bath, control the temperature at 0°C, and slowl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com