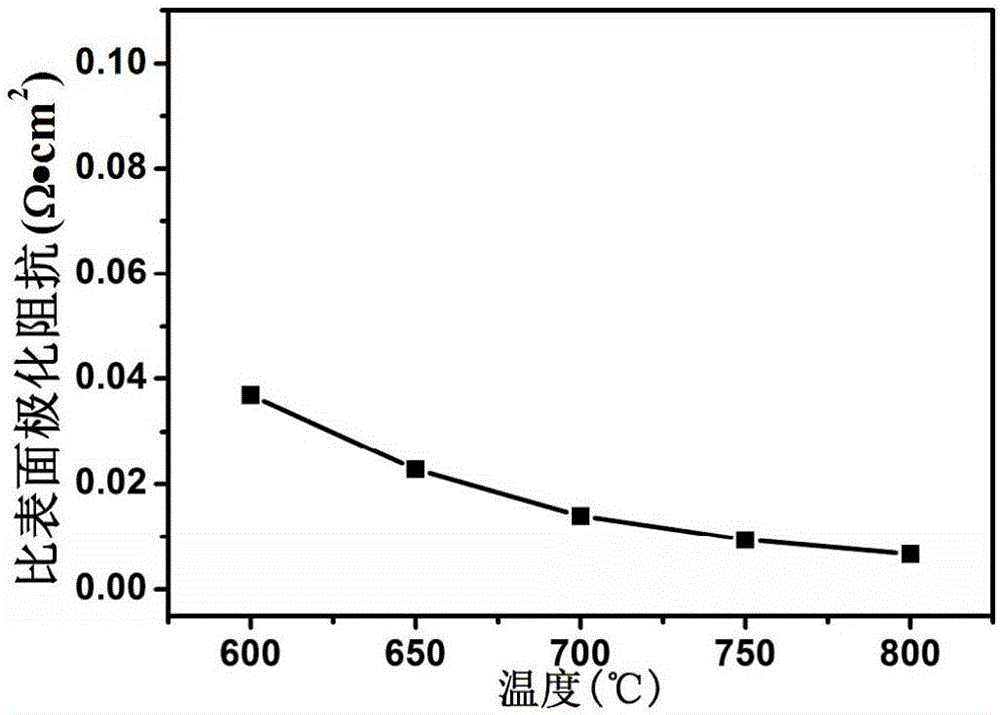
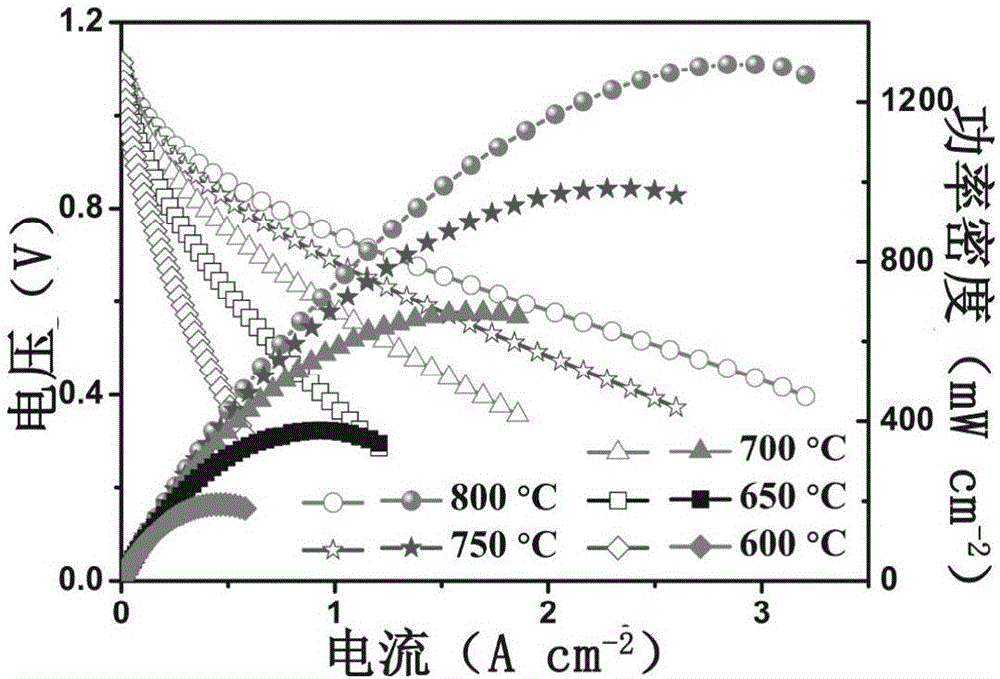
Ca-doping medium-low-temperature solid oxide fuel battery cathode material
A fuel cell cathode and solid oxide technology, which is applied to solid electrolyte fuel cells, battery electrodes, circuits, etc., can solve the problems of reduced conductivity and electrochemical performance, reduced thermal expansion coefficient, and high thermal expansion coefficient, so as to improve electrical conductivity, Reduced thermal expansion coefficient and excellent performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Example 1: PrBa 0.999 Ca 0.001 CoCuO 5+δ Powder preparation
[0019] Weigh 11.6869g of EDTA (analytical pure) and add it to deionized water, add ammonia water dropwise to adjust the pH of the solution to 6, and obtain mixed solution A; weigh 16.8112g of citric acid (analytical pure) and add it to deionized water, add ammonia water dropwise to adjust the pH of the solution value is 6, to obtain the mixed solution B; weigh Pr(NO 3 ) 3 ·6H 2 O (analytical pure) 4.3501g, Ba (NO 3 ) 2 (analytical pure) 2.6108g, Ca(NO 3 ) 2 4H 2 O (analytical pure) 0.00024g, Co (NO 3 ) 2 ·6H 2 O (analytical pure) 2.9103g and Cu (NO 3 ) 2 ·3H 2 O (analytical pure) 2.416g was dissolved in deionized water to obtain metal salt solution C; metal salt solution C and mixed solution B were added to mixed solution A, then heated and stirred in a water bath at 80°C for 12 hours, and evaporated to dryness to obtain a gel ; The resulting gel was treated at a low temperature of 250°C to ob...
Embodiment 2
[0020] Example 2: 80wt.% PrBa 0.4 Ca0.6 CoCuO 5+δ -20wt.%Gd 0.1 Ce 0.9 o 1.95 Powder preparation
[0021] Weigh 11.6869g of EDTA (analytical pure) and add it to deionized water, add ammonia water dropwise to adjust the pH of the solution to 6, and obtain mixed solution A; weigh 16.8112g of citric acid (analytical pure) and add it to deionized water, add ammonia water dropwise to adjust the pH of the solution value is 6, to obtain the mixed solution B; weigh Pr(NO 3 ) 3 ·6H 2 O (analytical pure) 4.3501g, Ba (NO 3 ) 2 (Analytical pure) 1.4635g, Ca(NO 3 ) 2 4H 2 O (analytical pure) 0.8502g, Co (NO 3 ) 2 ·6H 2 O (analytical pure) 2.9103g and Cu (NO 3 ) 2 ·3H 2 O (analytical pure) 2.4160g was dissolved in deionized water to obtain metal salt solution C; metal salt solution C and mixed solution B were added to mixed solution A, then heated and stirred in a water bath at 80°C for 12 hours, and evaporated to dryness to obtain a gel ; The resulting gel was treated at ...
Embodiment 3
[0024] Example 3 50wt.% PrCaCoCuO 5+δ -50wt.%Sm 0.2 Ce 0.8 o 1.9
[0025] Weigh 11.67g of EDTA (analytical pure) and add it to deionized water, add ammonia water dropwise to adjust the pH of the solution to 7, and obtain mixed solution A; weigh 16.8112g of citric acid (analytical pure) and add it to deionized water, add ammonia water dropwise to adjust the pH of the solution value is 7, to get the mixed solution B; weigh Pr(NO 3 ) 3 ·6H 2 O (analytical pure) 4.3501g, Ca (NO 3 ) 2 4H 2 O (analytical pure) 2.3616g, Co (NO 3 ) 2 ·6H 2 O (analytical pure) 2.9103g and Cu (NO 3 ) 2 ·3H 2 O (analytical pure) 2.4160g was dissolved in deionized water to obtain metal salt solution C; metal salt solution C and mixed solution B were added to mixed solution A, then heated and stirred in a water bath at 80°C for 12 hours, and evaporated to dryness to obtain a gel ; The obtained gel was treated at a low temperature of 350°C to obtain the battery cathode material—PrCaCoCuO 5+δ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com