Method for preparing azabicyalo compound by efficient catalysis of o-aminobenzenesulfonic acid and zirconocene Lewis acid
A technology of anthranilic acid and azabicyclo, which is applied in the field of synthesis of azabicyclic skeleton compounds, achieving the effects of high atom economy, short time and broad application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
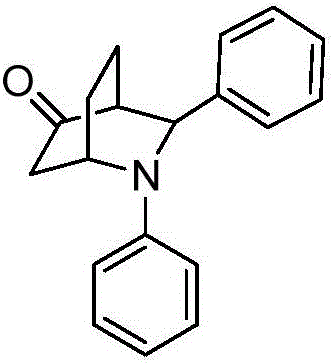
[0012] Preparation of 2,3-diphenyl-2-azabicyclo[2.2.2]cyclooctane-5-one with the following structural formula
[0013]
[0014] Add 0.0146 g (0.05 mmol) zirconocene dichloride, 0.0172 g (0.1 mmol) anthranilic acid, 100 μL (1.1 mmol) aniline, 102 μL (1 mmol) benzaldehyde, 194 μL (2 mmol) 2 - cyclohexen-1-one, 1 mL of absolute ethanol, stirred and reacted at 35 ° C for 4 hours, stopped the reaction, added 15 mL of ethyl acetate, removed ethyl acetate by rotary evaporation, and separated and removed zirconocene dichloride ( The eluent is a mixture of ethyl acetate and petroleum ether in a volume ratio of 1:5), to obtain 2,3-diphenyl-2-azabicyclo[2.2.2]cyclooctane-5-one, which The yield is 71%, and the dr value is 60:40. The resulting product is characterized by a Bruker Avance type superconducting Fourier digital nuclear magnetic resonance spectrometer, and the characterization data are: E configuration: 1 H NMR (400MHz, CDCl 3 )δ7.41(dt, J=15.0,7.6Hz,4H),7.31(t,J=6.9Hz,1H),...
Embodiment 2
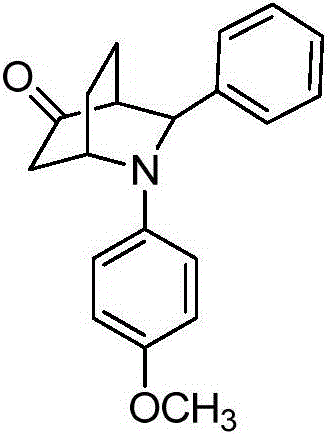
[0016] Preparation of 2-(4-methoxyphenyl)-3-phenyl-2-azabicyclo[2.2.2]cyclooctane-5-one with the following structural formula
[0017]
[0018] In Example 1, the aniline used is replaced with equimolar 4-methoxyaniline, and other steps are the same as in Example 1 to obtain 2-(4-methoxyphenyl)-3-phenyl-2-nitrogen Heterobicyclo[2.2.2]cyclooctan-5-one, its yield rate is 71%, dr value is 80:20, the characterization data of the obtained product is: E configuration: 1 H NMR (400MHz, CDCl 3 )δ7.45-7.35 (m, 4H), 7.29 (t, J = 7.1Hz, 1H), 6.74 (d, J = 9.0Hz, 2H), 6.56 (d, J = 9.1Hz, 2H), 4.71 ( s,1H),4.44(s,1H),3.70(s,3H),2.75(dd,J=12.2,9.4Hz,1H),2.66(d,J=2.8Hz,1H),2.38(d,J =18.7Hz,1H),1.94-1.84(m,1H),1.79-1.69(m,1H),1.66-1.57(m,2H); 13 C NMR (101MHz, CDCl 3 )δ 213.82, 152.07, 142.69, 140.46, 128.79, 127.37, 126.27, 114.89, 114.32, 62.67, 55.67, 51.07, 48.94, 41.95, 26.33, 16.35.
Embodiment 3
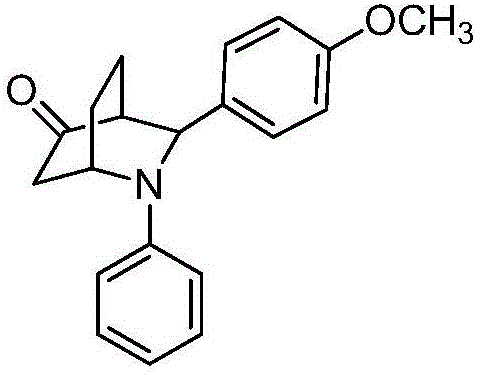
[0020] Preparation of 3-(4-methoxyphenyl)-2-phenyl-2-azabicyclo[2.2.2]cyclooctane-5-one with the following structural formula
[0021]
[0022] In Example 1, the benzaldehyde used is replaced with equimolar 4-methoxybenzaldehyde, and other steps are the same as in Example 1 to obtain 3-(4-methoxyphenyl)-2-phenyl-2 -Azabicyclo[2.2.2]cyclooctane-5-one, the yield is 72%, the dr value is 62:38, and the characterization data of the obtained product are: E configuration: 1 H NMR (400MHz, CDCl 3 )δ7.32(d, J=8.6Hz, 2H), 7.15(t, J=7.9Hz, 2H), 6.92(d, J=8.6Hz, 2H), 6.71(t, J=7.2Hz, 1H) ,6.61(d,J=8.5Hz,2H),4.73(s,1H),4.54(s,1H),3.82(s,3H),2.78-2.64(m,2H),2.40(d,J=18.0 Hz,1H),1.94-1.85(m,1H),1.78-1.58(m,3H); 13 C NMR (101MHz, CDCl 3 )δ 213.84, 158.89, 148.20, 131.77, 129.26, 127.16, 117.60, 114.19, 113.05, 61.82, 55.24, 51.13, 48.13, 42.21, 25.97, 16.25.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com