Preparation method of 2-methyl-4-amino-6-chloropyrimidine
A technology of methylpyrimidine and chloropyrimidine, which is applied in the field of preparation of 2-methyl-4-amino-6-chloropyrimidine, can solve problems such as poor economic benefits and environmental impact, difficult reactions, complicated operations, etc., and achieve simplification Reaction process and post-treatment process, reduce production cost, optimize the effect of preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
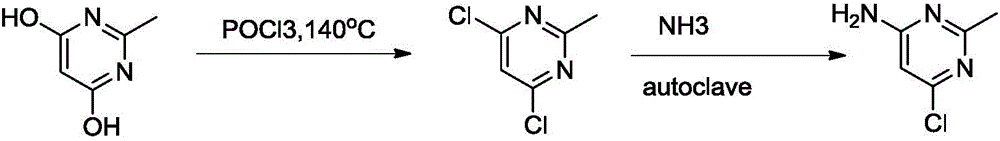
Image
Examples
Embodiment 1
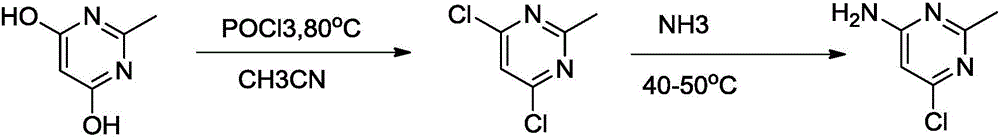
[0020] The first step: the synthesis of 4,6-dichloro-2-methylpyrimidine
[0021] 4,6-Dihydroxy-2-methylpyrimidine (5.0g, 0.04mol) was added to a mixed solution of phosphorus oxychloride (18.4g, 0.12mol) and acetonitrile, and the reaction was stirred at 60°C for 3 hours, and confirmed by spotting Raw material points disappear. The excess phosphorus oxychloride was distilled under reduced pressure, and the residue was poured into 50 g of ice water. The precipitated solid was filtered and purified by column to obtain 6 g (yield: 92%) of solid.
[0022] After nuclear magnetic detection, the nuclear magnetic spectrum is as follows, and the solid can be determined to be 4,6-dichloro-2-methylpyrimidine.
[0023] 1H NMR (CDCl3, 400MHz): d 2.63 (s, 3H, CH3), 7.17 [s, 1H, H (5)].
[0024] The second step: the synthesis of 2-methyl-4-amino-6-chloropyrimidine
[0025] Add 4,6-dichloro-2-methylpyrimidine (50g, 0.31mol) and ammonia water (0.5L) into a 1L reaction flask, stir at 40°C fo...
Embodiment 2
[0030] The first step: the synthesis of 4,6-dichloro-2-methylpyrimidine
[0031] 4,6-dihydroxy-2-methylpyrimidine (5.0g, 0.04mol) was added to a mixed solution of phosphorus oxychloride (12.26g, 0.08mol) and acetonitrile, and the reaction was stirred at 70°C for 3 hours, and confirmed by spotting Raw material points disappear. The excess phosphorus oxychloride was distilled under reduced pressure, and the residue was poured into 50 g of ice water. The precipitated solid was filtered and purified by column to obtain 5.8 g (yield: 90%) of solid.
[0032] Through nuclear magnetic detection, the solid can be determined to be 4,6-dichloro-2-methylpyrimidine.
[0033] The second step: the synthesis of 2-methyl-4-amino-6-chloropyrimidine
[0034] Add 4,6-dichloro-2-methylpyrimidine (50g, 0.31mol) and a mixed solution of ammonia water and tetrahydrofuran (0.5L) into a 1L reaction flask, stir at 45°C for 5 hours, cool the reaction to room temperature, filter, and use Wash with petro...
Embodiment 3
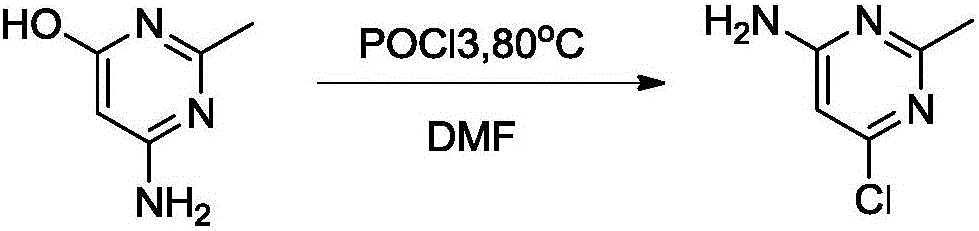
[0038] The first step: the synthesis of 4,6-dichloro-2-methylpyrimidine
[0039] 4,6-dihydroxy-2-methylpyrimidine (5.0g, 0.04mol) was added to a mixed solution of thionyl chloride (18.9g, 0.16mol) and acetonitrile, and the reaction was stirred at 80°C for 3 hours, and confirmed by spotting Raw material points disappear. The excess thionyl chloride was distilled under reduced pressure, and the residue was poured into 50 g of ice water. The precipitated solid was filtered and purified by column to obtain 6.1 g (yield: 94%) of solid.
[0040] Through nuclear magnetic detection, the solid can be determined to be 4,6-dichloro-2-methylpyrimidine.
[0041] The second step: the synthesis of 2-methyl-4-amino-6-chloropyrimidine
[0042] Add 4,6-dichloro-2-methylpyrimidine (50g, 0.31mol) and methanolic ammonia solution (0.5L) into a 1L reaction flask, stir at 50°C for 5 hours, cool the reaction to room temperature, filter, and use petroleum Wash with ether (100ml), filter, and dry und...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com