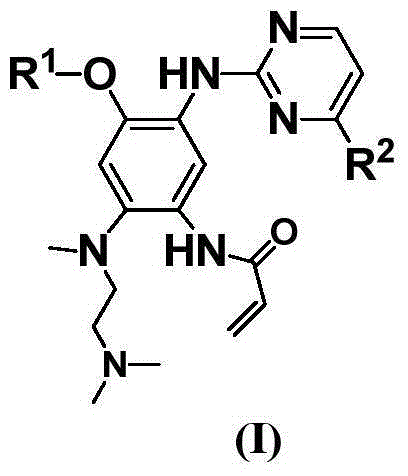
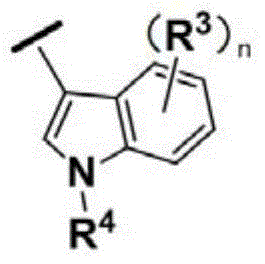
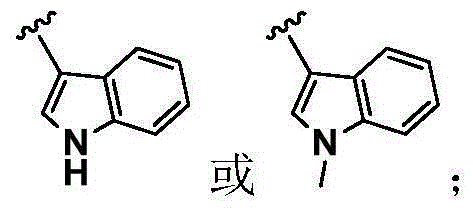
Pyrimidine compound as well as preparation method and medical application of pyrimidine compound
A compound, pyrimidine technology, applied in the field of chemical medicine, can solve the problems of poor selectivity, EGFR wild-type cytotoxicity and other problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 121
[0077]
[0078] 1. Synthesis of intermediate 121-3
[0079]
[0080] Under nitrogen protection, in a 100 milliliter (mL) three-necked flask at room temperature, the raw material 121-1 (3.0 grams (g), 25.6 mmol (mmol)) was dissolved in 50 mL 1,2-dichloroethane (DCE) , the mixture was lowered to 0°C, and methylmagnesium bromide (8.5mL, 25.6mmol) was added dropwise to the reaction system at 0°C. After the addition, the reaction was continued at constant temperature for 30 minutes (min), and then 121-2 (5.4 g, 36.3 mmol) was added into the reaction system, and the temperature was raised to room temperature to react overnight. After the reaction was complete, 100 ml of ice water was added to the reaction mixture to quench the reaction, the mixture was extracted 3 times with 100 ml of dichloromethane, the combined organic phases were washed 3 times with 100 ml of saturated brine, dried over anhydrous sodium sulfate and concentrated. The crude product was purified by silica ge...
Embodiment 122
[0113]
[0114] 1. Synthesis of intermediate 122-1
[0115]
[0116]Under nitrogen protection, add 5-fluoroindole (10g, 74.0mmol) into a 500mL three-necked flask, distill 100mL of anhydrous tetrahydrofuran, lower the temperature to 0°C, and add 37.1mL of methylmagnesium bromide ether solution (3.0M ), after the dropwise addition, keep at 0°C for about 30min, add 2,4-dichloropyrimidine (16.5g, 111mmol) in batches at 0°C, and return the reaction system to room temperature to react overnight. After detecting that the reaction is complete, add 100 mL of ammonium chloride aqueous solution dropwise to the reaction system to quench the reaction, extract the resulting mixture twice with 100 mL of ethyl acetate, combine the organic phases, backwash once with 100 mL of saturated brine, and then wash with anhydrous sulfuric acid. After sodium drying and concentration to dryness, the obtained solid was washed once with 100 mL of acetonitrile, filtered with suction, and the filter ca...
Embodiment 123
[0141]
[0142] 1. Synthesis of intermediate 123-1
[0143]
[0144] Under the protection of nitrogen, the raw material 122-1 (2.5 g, 10.1 mmol) was dissolved in 50 mL of anhydrous DMF in a 100 mL three-necked flask at room temperature, and then the temperature was lowered to 0 °C and NaH (65%, 560 mg, 15.2 mmol) was added in batches, After the addition, the reaction system was kept at 0°C for 30 minutes. Then 1,1-difluoro-2-bromoethane (2.9 g, 20.0 mmol) was added dropwise at 0° C. After the dropwise addition, the system was returned to room temperature to react overnight. After detecting that the reaction was complete, the reaction mixture was poured into 200 mL of ice water to quench the reaction, and a solid was precipitated. The mixture was filtered to collect the filter cake, washed once with 50 mL of acetonitrile, and dried to obtain 1.6 g (51%) of product 123-1. It is a yellow solid. LCMS: 312.0.
[0145] 2. Synthesis of Compound 123
[0146]
[0147] The ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com