Chemochromic nanoparticles, method of making the same, and hydrogen sensor including the same
A nanoparticle, chemical color change technology, applied in the field of chemical color change nanoparticles, can solve the problems of reduced reproducibility, hydrogen sensor can not be used as a substitute for existing sensors, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
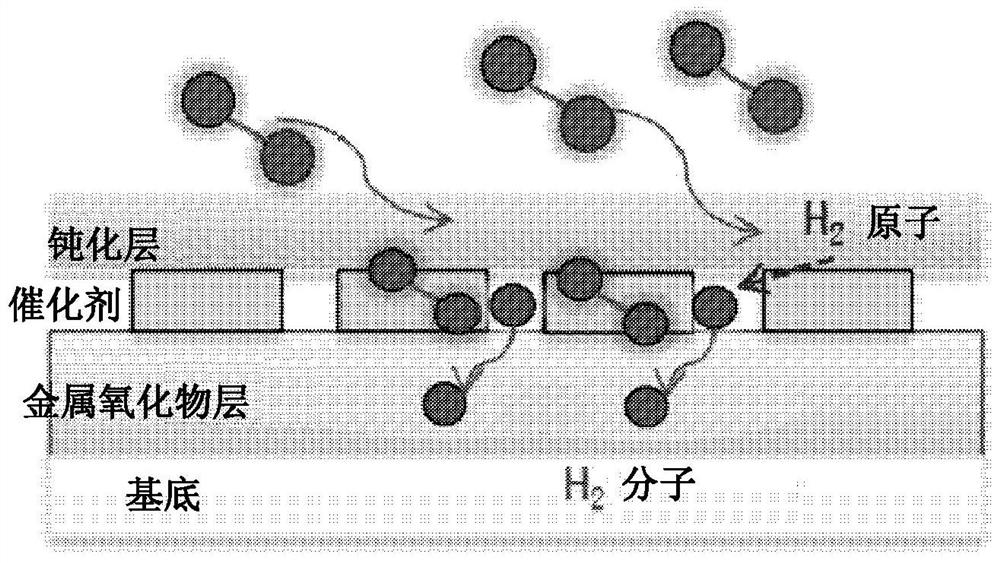
[0057] In detail, according to an exemplary embodiment of the present invention, there is provided a chemichromic nanoparticle that may have a core-shell structure, such that the chemichromic nanoparticle may include a core including a hydrated or non-hydrated transition metal oxide; and a partially coated A shell comprising a metal catalyst covers the surface of the core. Preferably, the shell may fully or partially coat the surface of the core. For example, the shell can coat about 10%, 20%, 30%, 40%, 50%, 60%, 70%, 80%, 90%, 95%, or 99% of the total surface area of the core.
[0058] The transition metal oxide used for the nanoparticle core may be a material whose color changes chemically when the material is exposed to hydrogen gas due to reduction by reaction with hydrogen molecules. Representative examples thereof may include one or two or more selected from SnO 2 、TiO 2 , ZnO, VO 2 、In 2 o 3 , NiO, MoO 3 , SrTiO 3 , Fe 2 o 3 、WO 3 and CuO metal oxides. Pre...
Embodiment
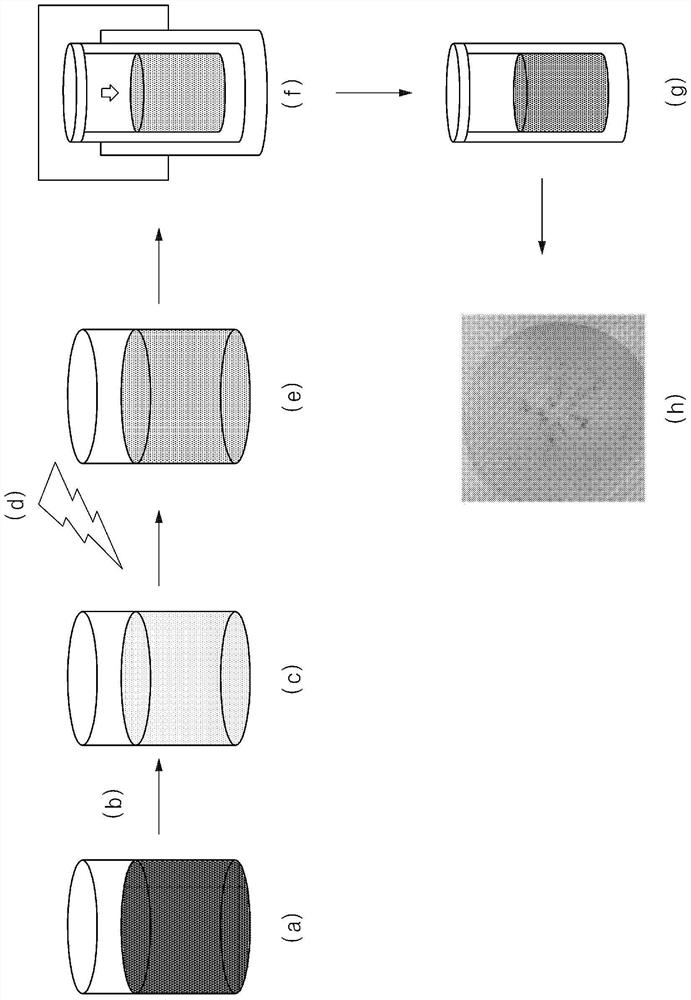
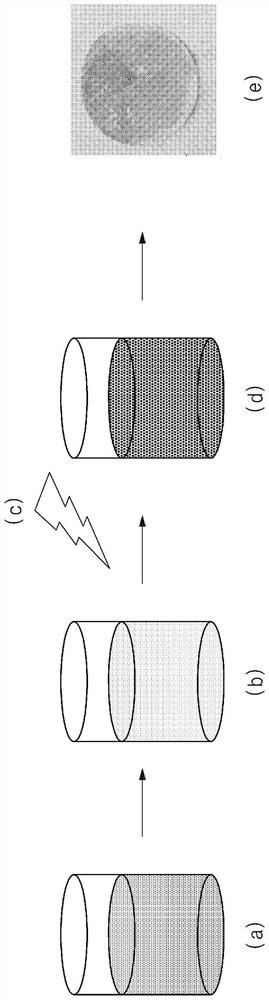
[0107] Experimental method and apparatus
[0108] a. Observe 1% hydrogen in a mixed atmosphere of nitrogen, oxygen and water vapor (at 99% air balance gas (N 2 、H 2 O, O 2 ) in) the color change reaction.
[0109] b. All aerochromic experiments were performed at room temperature, and a flow rate of 2 L / min was maintained on the sample.
preparation example 1
[0110] Preparation Example 1: Preparation of Hydrated Tungsten Oxide
[0111] After preparing a 1 wt % ammonium paratungstate aqueous solution by mixing ammonium paratungstate and water in a reactor, an aqueous tungsten solution was prepared by adding 1.5 ml of hydrochloric acid (HCl) thereto while stirring the aqueous solution, and stirring the mixed solution for another 30 minutes.
[0112] Then, 3 ml of hydrogen peroxide was added to the aqueous tungsten solution, and stirred at room temperature for 60 minutes until the mixed solution became transparent, thereby preparing an aqueous solution of peroxy-polytungstic acid.
[0113] The peroxy-polytungstic acid aqueous solution was injected into the autoclave in which the internal pressure was maintained at 35-50 bar, and it was subjected to the first heat treatment at a temperature of 160° C. for about 1.5 hours.
[0114] After the reaction was terminated, the autoclave was air-cooled to room temperature, and the precipitate...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com