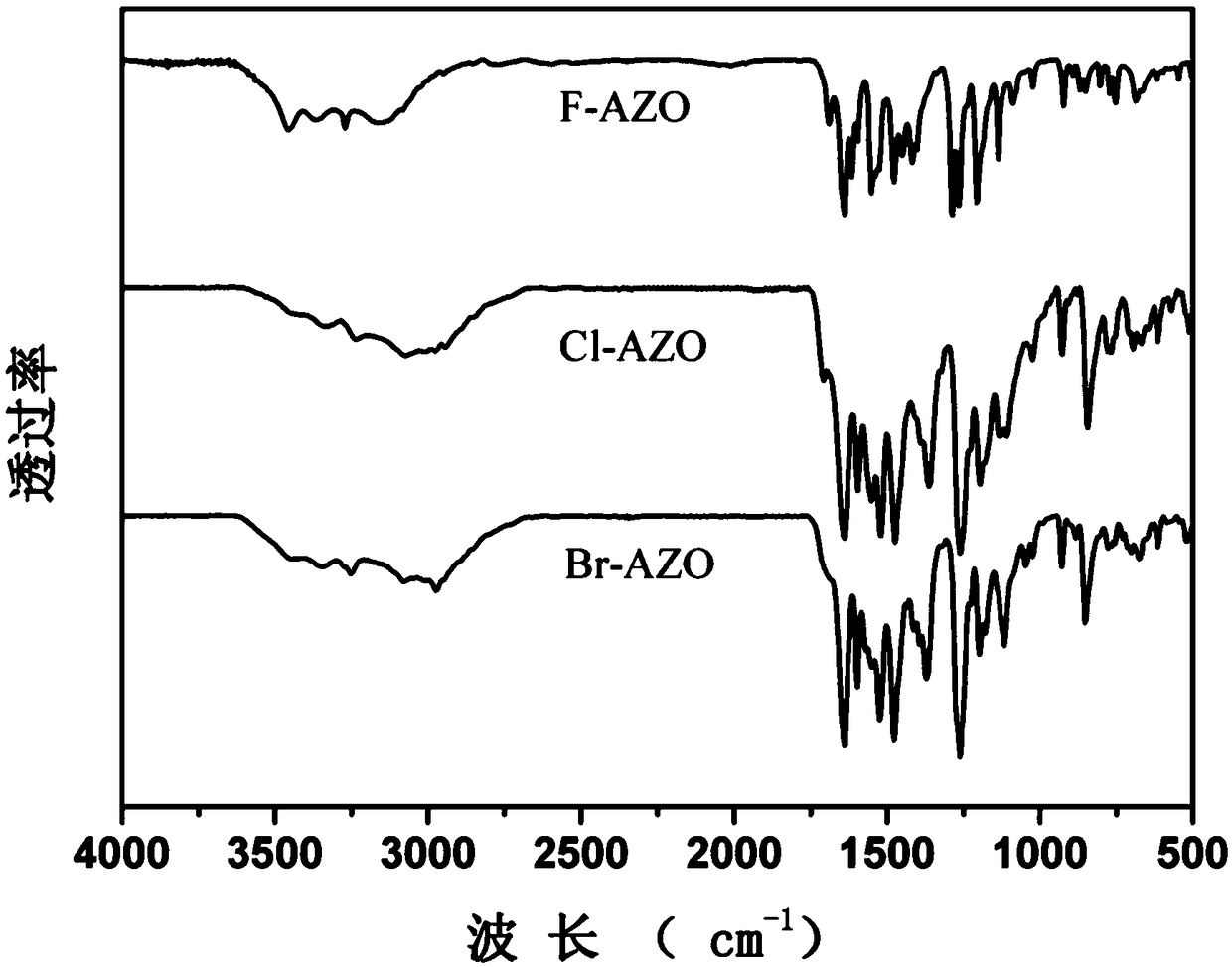
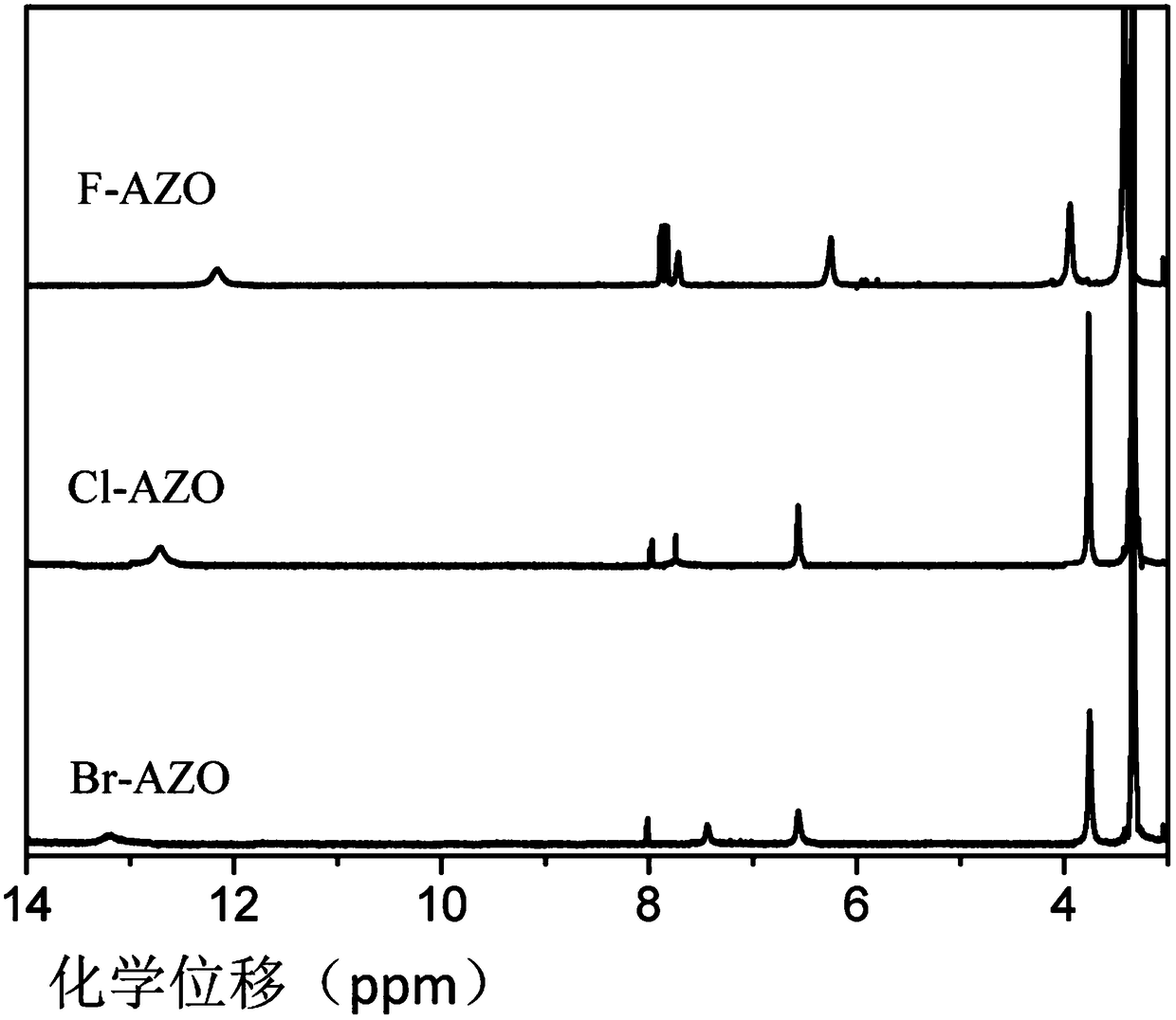
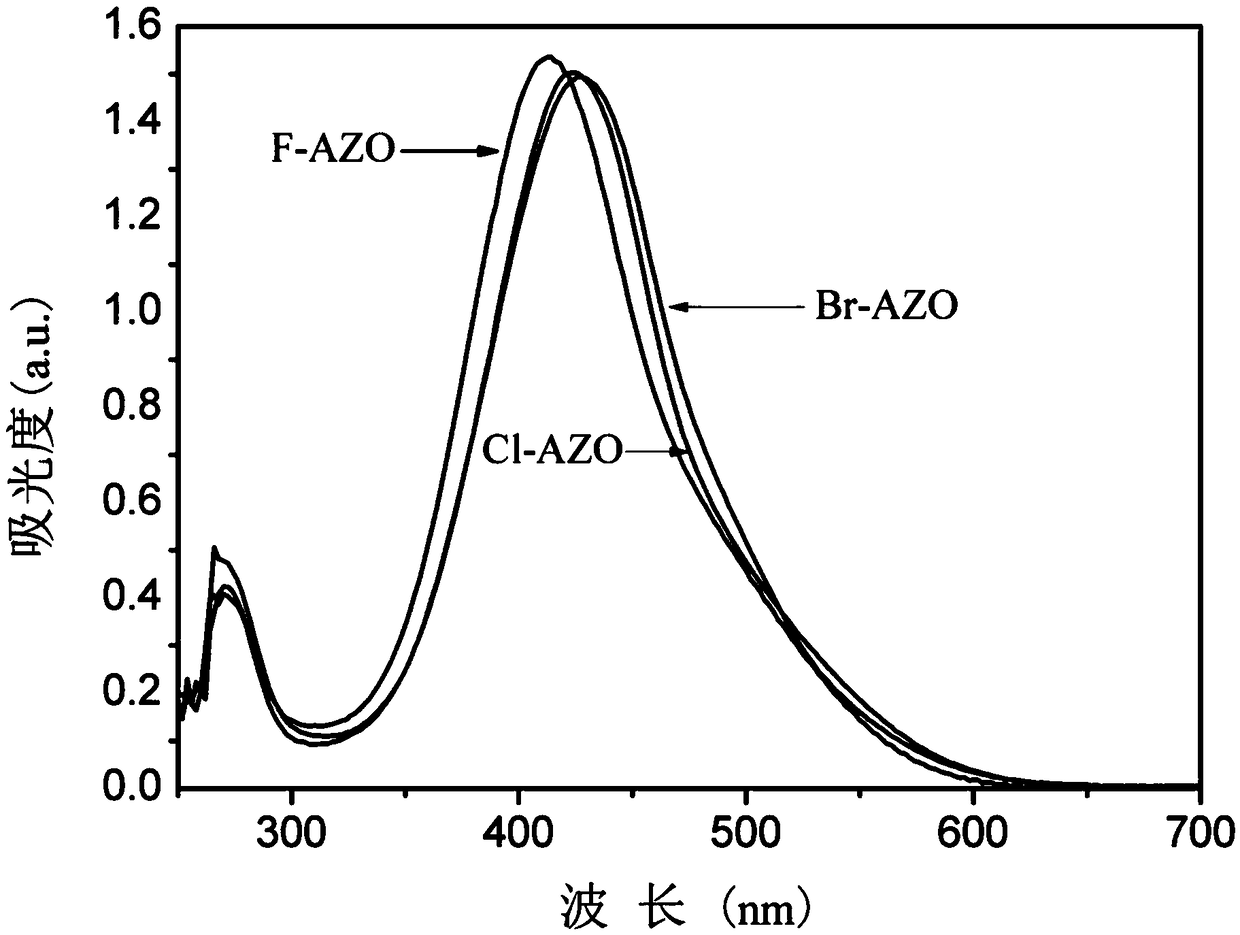
A class of ortho-positioned azobenzene derivatives containing charge-withdrawing groups for solar thermal storage and preparation method thereof
An azobenzene derivative, solar thermal technology, applied in heat exchange materials, chemical instruments and methods, organic chemistry, etc., can solve the problems of reduced energy density, large steric hindrance, short service life, etc. , the preparation method is simple, the effect of high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0031] 1) Preparation of diazonium salt: prepare 0.3-0.6 mol / L 4-amino-3-fluoro (chloro, bromo) benzoic acid aqueous solution in a beaker, and mix a 37.5% HCl solution (HCl with the moles of the above-mentioned drugs) in a beaker ratio of 1:4) into it, and kept stirring until the 4-amino-3-fluoro(chloro, bromo)benzoic acid was fully dissolved, and it was kept in an ice bath (0-5°C); weighed NaNO 2 (1:1 molar ratio to 4-amino-3-fluoro(chloro, bromo)benzoic acid) was dissolved in distilled water and poured into the above solution; kept stirring under ice bath conditions to obtain a diazonium salt solution.
[0032] 2) Coupling reaction of diazonium salt and 3,5-dimethoxyaniline: weigh 3,5-dimethoxyaniline (1:1 molar ratio to diazonium salt) in distilled water; drop into beaker Add the HCl solution that mass fraction is 37.5%, (HCl and above-mentioned drug molar ratio are 1:4) edge dropwise stirring, until completely dissolving and keep it under ice-bath condition; And above-men...
Embodiment 1
[0036] 1) Preparation of diazonium salt: weigh 4-amino-3-fluorobenzoic acid of 0.93g weight in a beaker and pour 20ml of distilled water into it, and measure 2ml of 37.5% HCl solution in mass fraction, without Stir continuously until the 4-amino-3-fluorobenzoic acid is fully dissolved, and keep it in an ice bath (0-5°C); weigh 0.40g of NaNO 2 In a beaker, add 10ml of distilled water to dissolve completely and pour it into the above solution; keep stirring for 1h under ice bath conditions to obtain a diazonium salt solution.
[0037] 2) Coupling reaction of diazonium salt and 3,5-dimethoxyaniline: Weigh 0.92 g of 3,5-dimethoxyaniline (1:1 molar ratio to diazonium salt) in a beaker, Pour 50ml of distilled water into the volume; dropwise add 2ml of concentrated HCl solution with a mass fraction of 37.5% in the beaker, stir while adding dropwise, until completely dissolved and keep it under ice bath conditions; and the above-mentioned diazonium salt solution Slowly added dropwise...
Embodiment 2
[0042]1) Preparation of diazonium salt: weigh 4-amino-3-chlorobenzoic acid of 1.02g weight in a beaker and pour 20ml of distilled water into it, and measure 2ml of HCl solution with a mass fraction of 37.5%. Stir continuously until the 4-amino-3-chlorobenzoic acid is fully dissolved, and keep it in an ice bath (0-5°C); weigh 0.40g of NaNO 2 In a beaker, add 10ml of distilled water to dissolve completely and pour it into the above solution; keep stirring for 1h under ice bath conditions to obtain a diazonium salt solution.
[0043] 2) Coupling reaction of diazonium salt and 3,5-dimethoxyaniline: Weigh 0.92 g of 3,5-dimethoxyaniline (1:1 molar ratio to diazonium salt) in a beaker, Pour 50ml of distilled water into the volume; dropwise add 2ml of concentrated HCl solution with a mass fraction of 37.5% in the beaker, stir while adding dropwise, until completely dissolved and keep it under ice bath conditions; and the above-mentioned diazonium salt solution Slowly added dropwise t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com