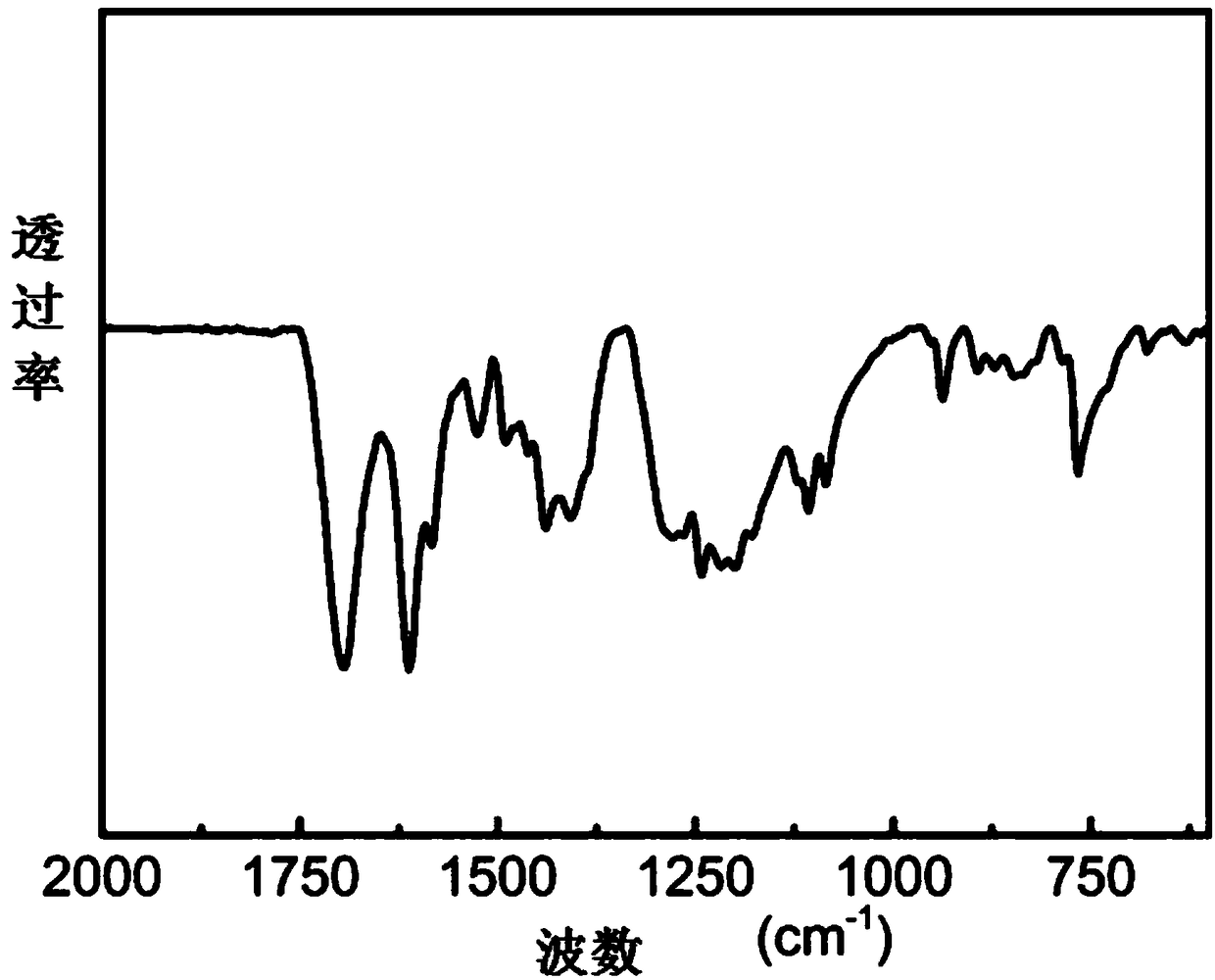
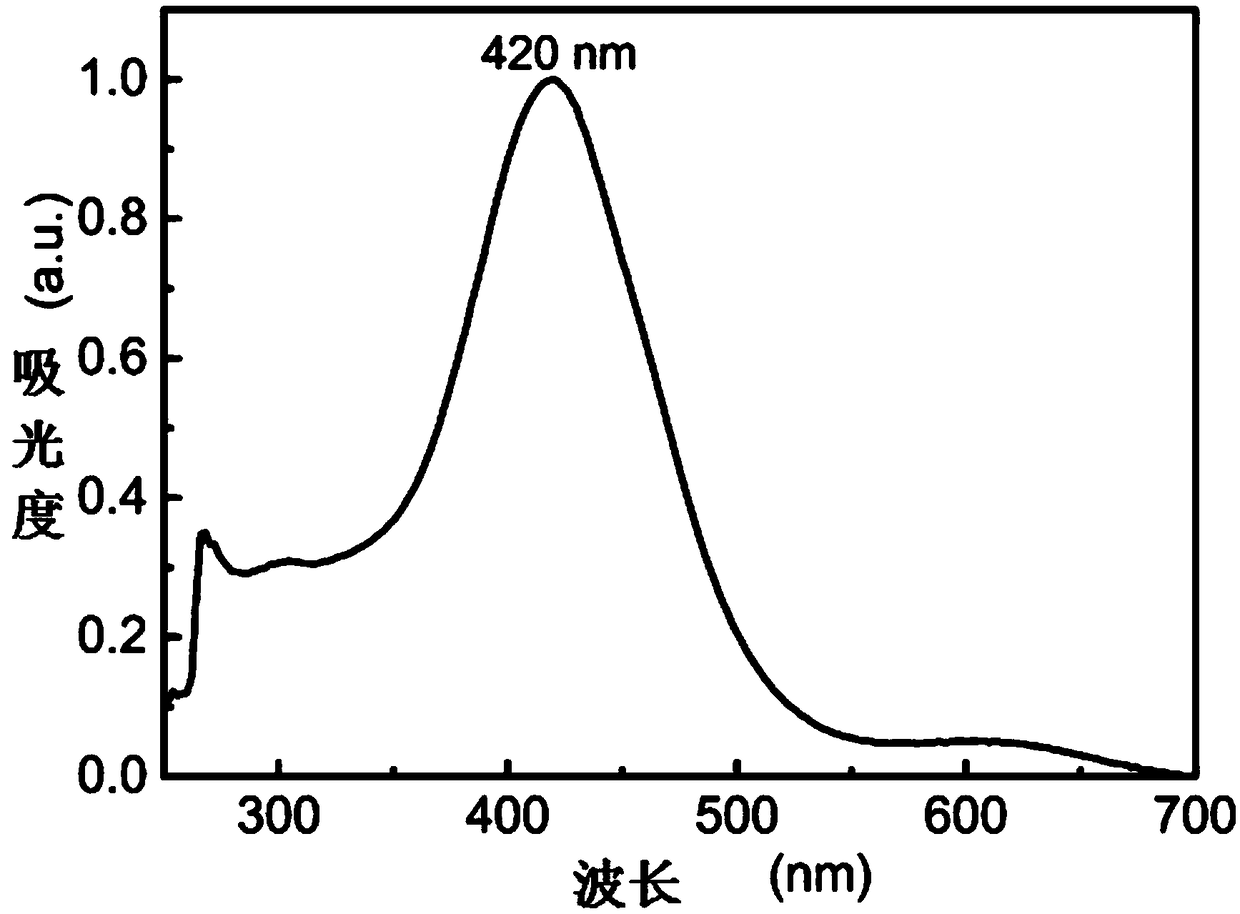
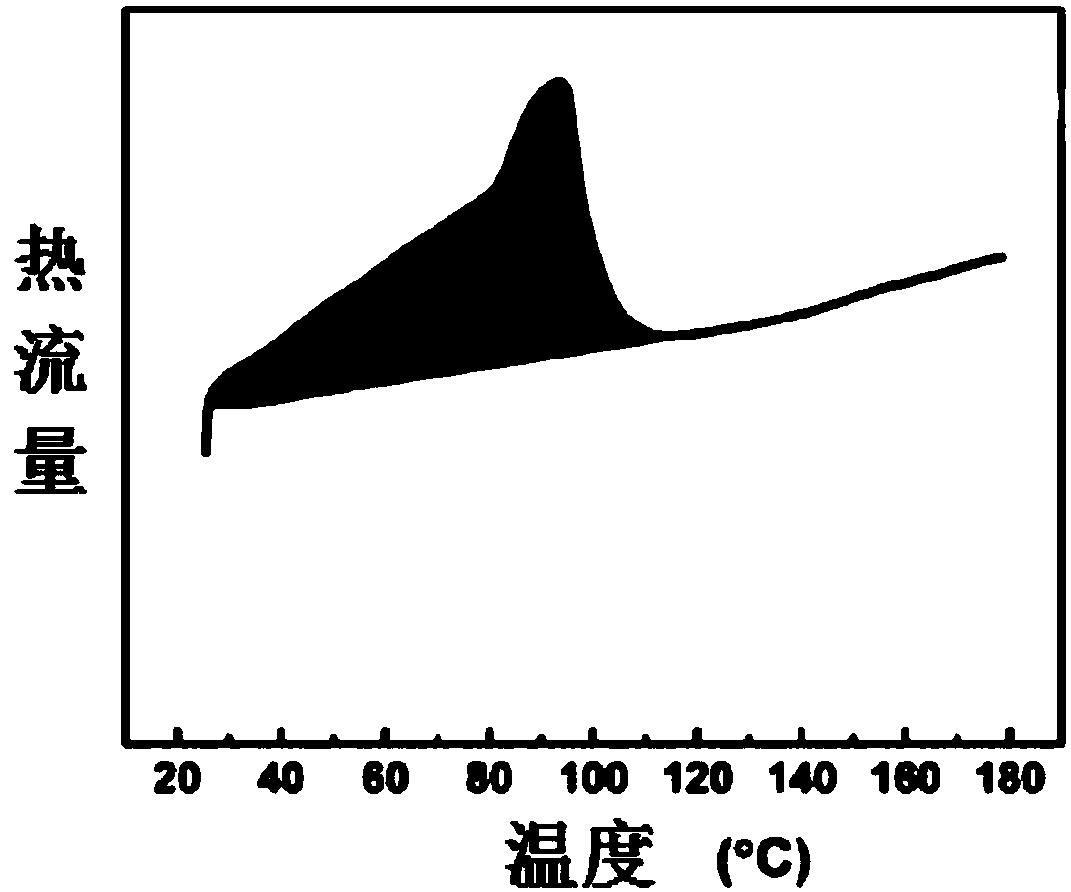
Ortho-fluoro-azobenzene derivative and preparation method thereof
An azobenzene derivative and ortho-position technology is applied in the field of preparation of ortho-fluorinated azobenzene, which can solve the problems of limiting the application range of azobenzene, easy scattering of ultraviolet rays, biological damage, etc., so as to prolong the recovery half-life, Excellent optical performance, the effect of improving stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] 1) Preparation of diazonium salt: 1.55g of 4-amino-3-fluorobenzoic acid (10mmol) and 0.4g of NaOH (10mmol) were weighed, dissolved in 30mL of deionized water, and recorded as A. Weigh 0.76g NaNO 2 (11mmol) was dissolved in 10mL deionized water, and 10mL NaNO 2 Slowly added dropwise to A. After being completely dissolved, the solution was added dropwise to 40mL HCl (1mol L -1 ), keep the temperature at 0-5°C, stir and react for 60 minutes to obtain a diazonium salt solution, and store in an ice bath.
[0032] 2) Coupling reaction: weigh 1.11g 3-fluoroaniline (10mmol) (1:1 molar ratio to diazonium salt) and dissolve in HCl (1mol L -1 ), denoted as B; and the above-mentioned diazonium salt solution was slowly added dropwise to B and stirred.
[0033] 3) Adjust pH: prepare NaHCO 3 Saturated aqueous solution; add the aqueous solution dropwise to the above mixed solution to adjust the pH, and adjust the pH to 5-7; continue to stir for 5 hours under ice bath conditions, a...
Embodiment 2
[0036] 1) Preparation of diazonium salt: 3.1 g of 4-amino-3-fluorobenzoic acid (20 mmol) and 0.8 g of NaOH (20 mmol) were weighed, dissolved in 100 mL of deionized water, and recorded as A. Weigh 1.52g NaNO 2 (22mmol) was dissolved in 20mL deionized water, and 20mL NaNO 2 Slowly added dropwise to A. After being completely dissolved, the solution was added dropwise to 80mL HCl (1mol L -1 ), keep the temperature at 0-5°C, stir and react for 60 minutes to obtain a diazonium salt solution, and store in an ice bath.
[0037] 2) Coupling reaction: Weigh 2.22g of 3-fluoroaniline (20mmol) (the molar ratio of diazonium salt is 1:1) and dissolve it in HCl, denoted as B; slowly add the above diazonium salt solution dropwise Stir in B.
[0038] 3) Adjust pH: prepare NaHCO 3 Saturated aqueous solution; add the aqueous solution dropwise to the above mixed solution to adjust the pH, and adjust the pH to 5-7; continue to stir for 8 hours under ice bath conditions, and filter under reduced ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com