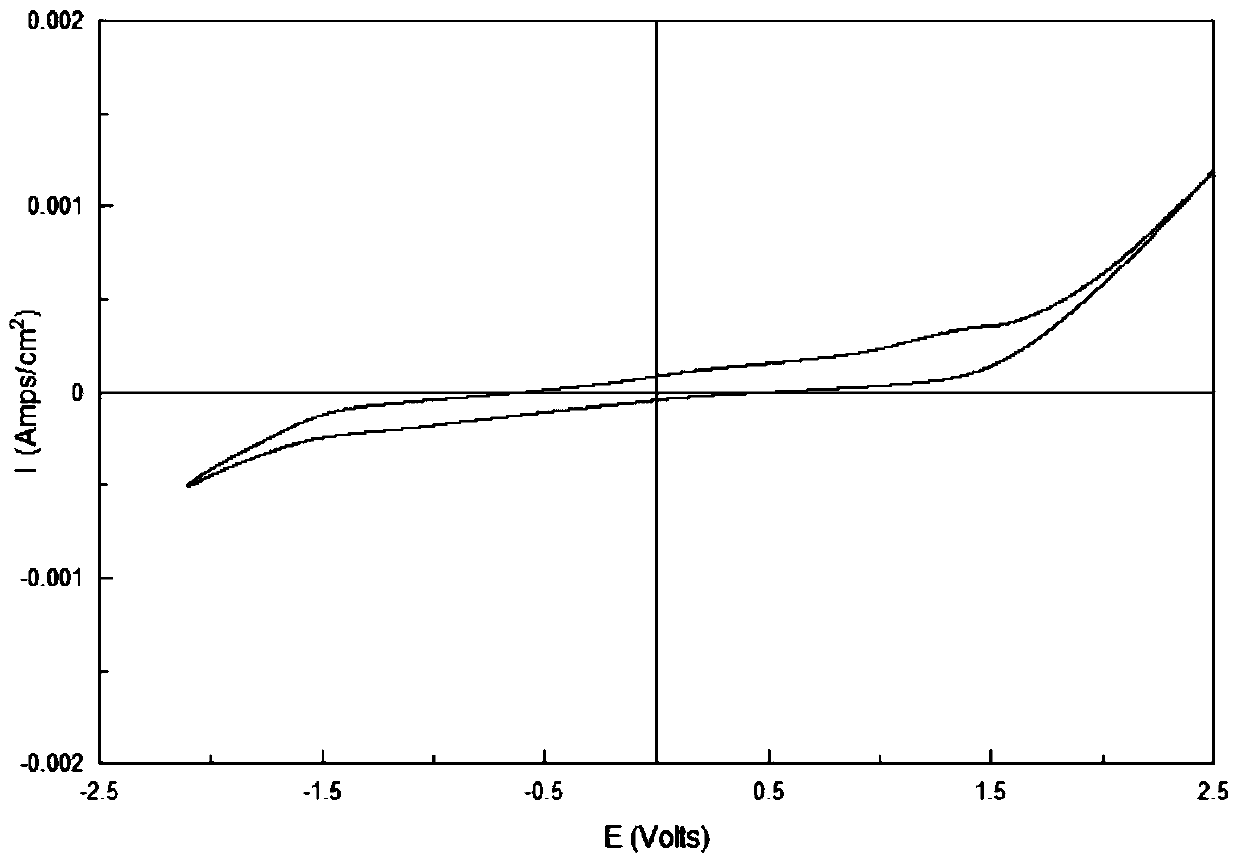
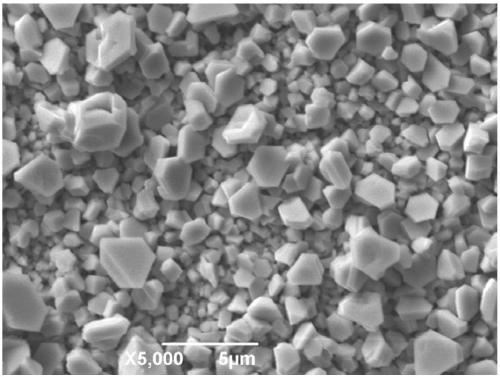
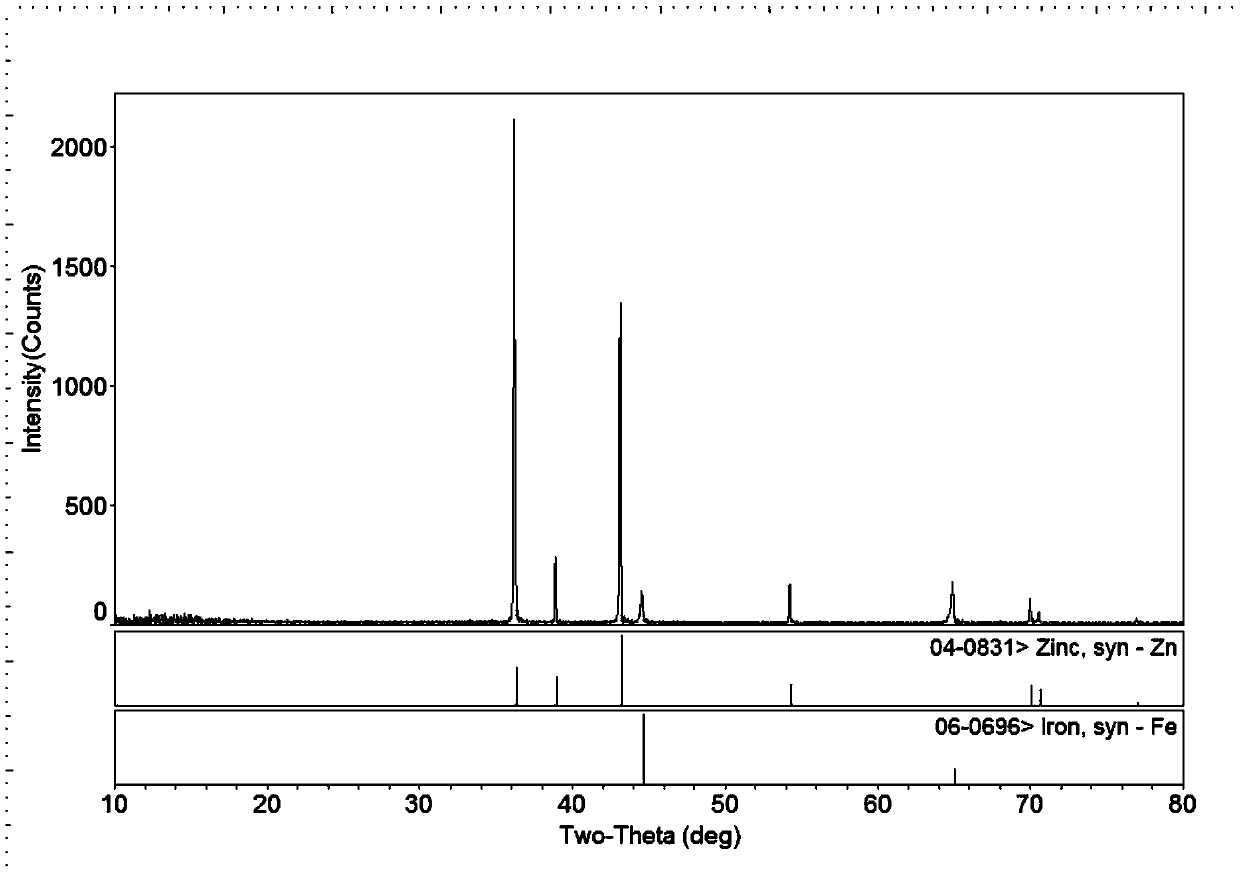
A kind of electrogalvanizing method based on betaine-urea-water deep eutectic solvent
A technology of deep eutectic solvent and betaine, which is applied in the field of electroplating, can solve problems such as equipment corrosion and pollution, and achieve the effect of reducing corrosion potential
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] First weigh 0.2 mol of betaine, 0.4 mol of urea, and 0.6 mol of distilled water and place them in a beaker, seal the beaker with plastic wrap, and then stir at a constant temperature of 80°C to obtain a transparent deep eutectic solvent. Then add 0.02 mol of zinc chloride and stir at a constant temperature of 80° C. to obtain a zinc-containing deep eutectic solvent.
[0031] Use a pure zinc plate as the anode, and low-carbon steel as the cathode, immerse in a zinc-containing eutectic solvent heated to 50°C in a water bath for 30 minutes of constant current electroplating, in which the direct distance between the cathode and the anode is 1 cm, and the current density is 4 mA / cm 2 .
[0032] After the electroplating is completed, the low carbon steel is taken out and washed twice with double distilled water, and finally dried in an oven at 60°C.
[0033] The conductivity of betaine-urea-water deep eutectic solvent is 33.4μs cm at 50℃ -1 .
[0034] The conductivity of t...
Embodiment 2
[0036] First weigh 0.2 mol of betaine, 0.4 mol of urea, and 0.6 mol of distilled water and place them in a beaker, seal the beaker with plastic wrap, and then stir at a constant temperature of 80°C to obtain a transparent deep eutectic solvent. Then add 0.025 mol of zinc chloride, and stir at 80° C. to obtain a zinc-containing deep eutectic solvent.
[0037] Use a pure zinc plate as the anode, and low-carbon steel as the cathode, immerse in a zinc-containing eutectic solvent heated to 60°C in a water bath for 15 minutes of constant current electroplating, in which the direct distance between the cathode and the anode is 2cm, and the current density is 2mA / cm 2 .
[0038] After the electroplating is completed, the low carbon steel is taken out and washed twice with double distilled water, and finally dried in an oven at 60°C.
Embodiment 3
[0040] First weigh 0.2 mol of betaine, 0.4 mol of urea, and 0.6 mol of distilled water and place them in a beaker, seal the beaker with plastic wrap, and then stir at a constant temperature of 80°C to obtain a transparent deep eutectic solvent. Then add 0.02 mol of zinc chloride and stir at a constant temperature of 80° C. to obtain a zinc-containing deep eutectic solvent.
[0041] Use a pure zinc plate as the anode, and low-carbon steel as the cathode, immerse in a zinc-containing eutectic solvent heated to 70°C in a water bath for 15 minutes of constant current electroplating, in which the direct distance between the cathode and the anode is 1cm, and the current density is 6mA / cm 2 .
[0042] After the electroplating is completed, the low carbon steel is taken out and washed twice with double distilled water, and finally dried in an oven at 60°C.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com