Preparation method of flaky Cu9Fe9S16 nanoflowers
A technology of cu9fe9s16 and nanoflowers, which is applied in the preparation and application of light-to-heat conversion materials, can solve the problems of high temperature in pyrolysis, and achieve the effect of easy availability of raw materials, low price and environmental friendliness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
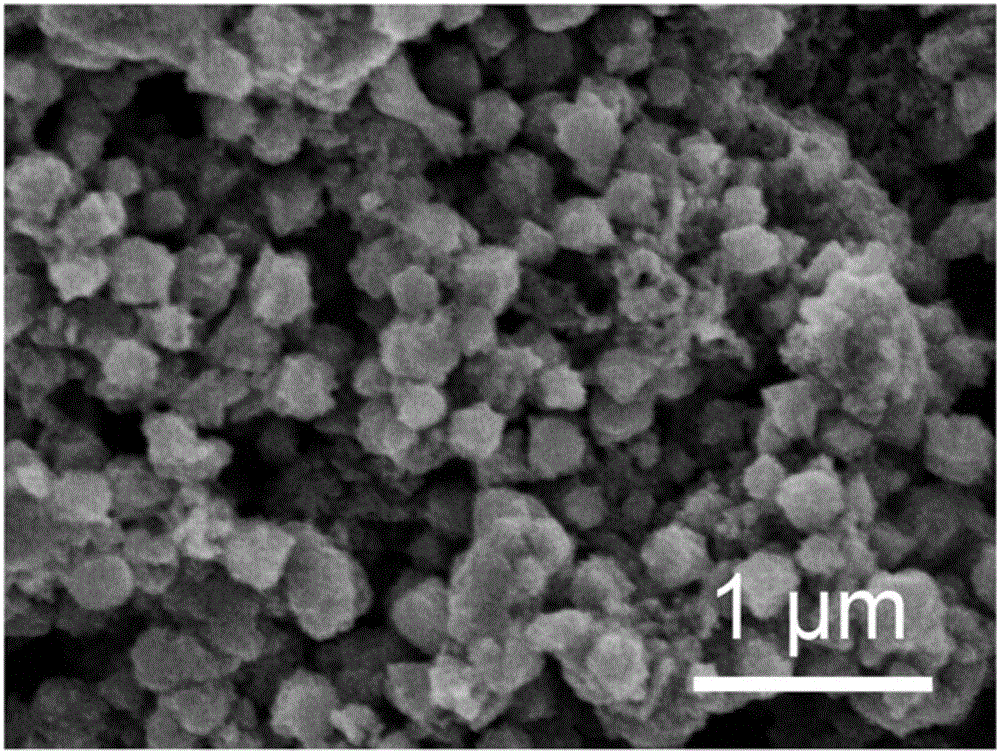
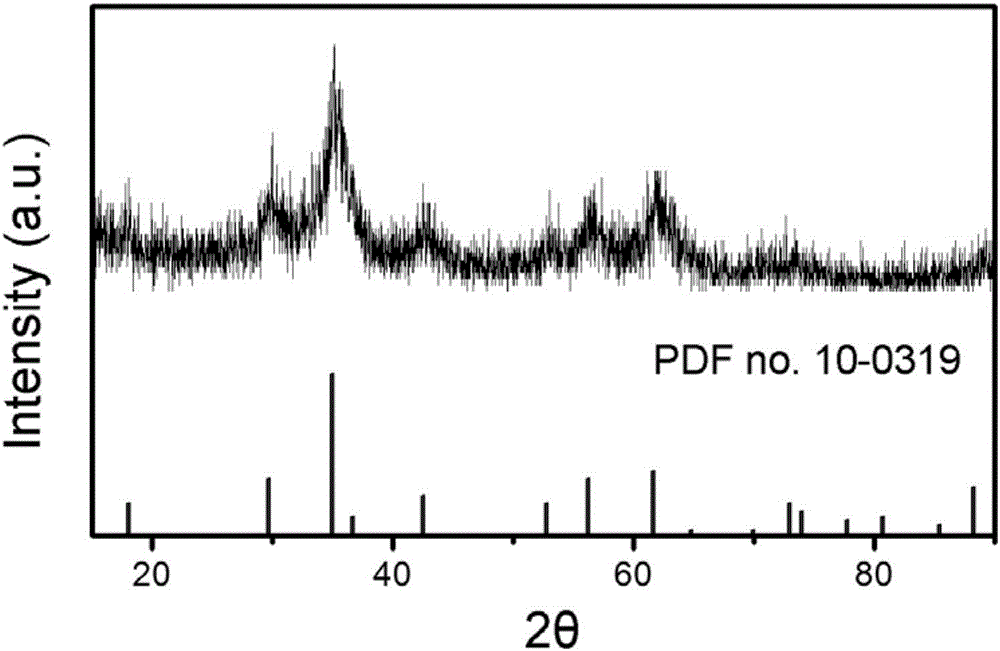
[0033] Take 0.67mmol FeCl 3 ·6H 2 O and 0.33 mmol MnCl 2 4H 2 Disperse O in 10mL of diethylene glycol, stir at 80°C, add 0.4mmol trisodium citrate dihydrate, stir for 1h, add 3mmol sodium acetate, after 30min, transfer to a polytetrafluoroethylene reactor, 200°C Under the reaction 6h. Centrifuged and washed 3 times with deionized water to obtain Fe 2 MnO 4 Nanocrystalline. like figure 1 Shown is the scanning electron microscope image of the sample, the particle size is 200–400nm. figure 2 is the XRD picture of the sample, with Fe 2 MnO 4 (PDFno.10-0319) standard XRD peak one-to-one correspondence, indicating that the prepared sample is Fe 2 MnO 4 .
[0034] Fe obtained by taking 2.5mg 2 MnO 4 Nanocrystals, dispersed in 10mL water, add 10μL (NH 4 ) 2 S solution (22wt%), ultrasonically dispersed for 5min. Centrifuge, wash with deionized water 3 times, and disperse in 10 mL of water.
[0035] Take 2mL CuCl 2 The dispersion liquid (5.425mmol / L) was added to th...
Embodiment 2
[0038] Take 0.67mmol FeCl 3 ·6H 2 O and 0.33 mmol MnCl 2 4H 2 Disperse O in 20mL diethylene glycol, stir at 80°C, add 0.4mmol trisodium citrate dihydrate, stir for 1h, add 3mmol sodium acetate, after 30min, transfer to a polytetrafluoroethylene reactor, 240°C Under the reaction 6h. Centrifuged and washed 3 times with deionized water to obtain Fe 2 MnO 4 Nanocrystalline.
[0039] Fe obtained by taking 2.5mg 2 MnO 4 Nanocrystals, dispersed in 10mL water, add 10μL (NH 4 ) 2 S solution (22wt%), ultrasonically dispersed for 8min. Centrifuge, wash with deionized water 3 times, and disperse in 10 mL of water.
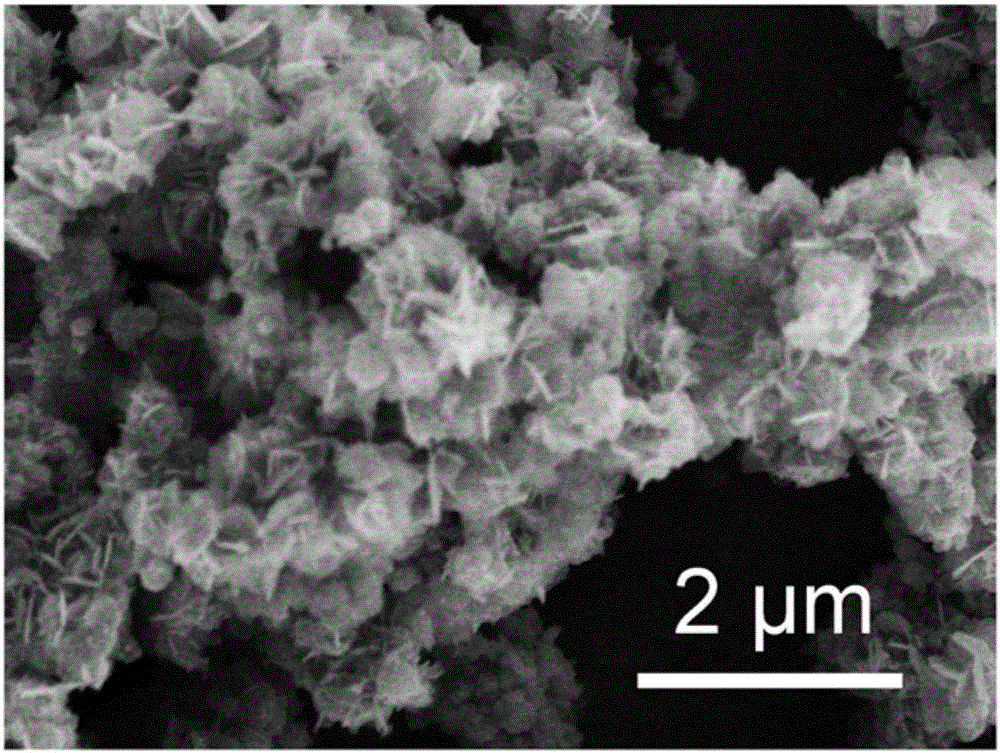
[0040] Take 2mL CuCl 2 The dispersion liquid (5.425mmol / L) was added to the dispersion liquid obtained above, and ultrasonically dispersed for 8min. Centrifuged and washed 3 times with deionized water to obtain flake Cu 9 Fe 9 S 16 nanoflowers.
Embodiment 3
[0042] Take 0.67mmol FeCl 3 ·6H 2 O and 0.33 mmol MnCl 2 4H 2 Disperse O in 10mL of diethylene glycol, stir at 80°C, add 0.4mmol trisodium citrate dihydrate, stir for 1h, add 3mmol sodium acetate, after 30min, transfer to a polytetrafluoroethylene reactor, 200°C Under the reaction 6h. Centrifuged and washed 3 times with deionized water to obtain Fe 2 MnO 4 Nanocrystalline.
[0043] Fe obtained by taking 2.5mg 2 MnO 4 Nanocrystals, dispersed in 10mL water, add 20μL (NH 4 ) 2 S solution (22wt%), ultrasonically dispersed for 10 minutes. Centrifuge, wash with deionized water 3 times, and disperse in 10 mL of water.
[0044] Take 2mL CuCl 2 The dispersion liquid (5.425mmol / L) was added to the dispersion liquid obtained above, and ultrasonically dispersed for 5min. Centrifuged and washed 3 times with deionized water to obtain flake Cu 9 Fe 9 S 16 nanoflowers.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com