Method for synthesizing biaryl
A biaryl hydrocarbon and halogenated hydrocarbon technology, applied in the field of organic catalysis synthesis, can solve the problems of polluting the environment, high reaction cost, difficult to achieve large-scale production, etc., and achieve the effects of low corrosiveness, less dosage and easy preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
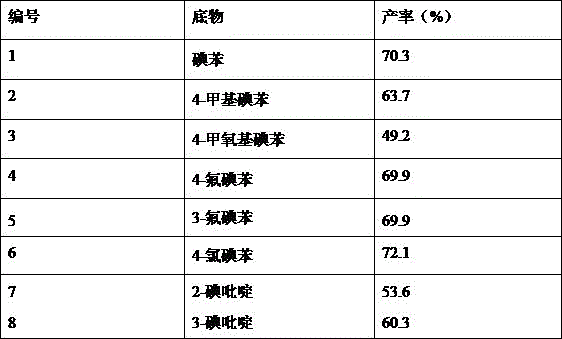
Examples
Embodiment 1
[0012] 1. Prepare the catalyst:
[0013] Weigh 1231.6 mg (10 mol) of p-methoxyaniline and dissolve it in 100 ml of aqueous hydrochloric acid with a concentration of 1 mol / L to obtain solution a.
[0014] Weigh 44.3 mg of palladium dichloride and dissolve in 100 ml of aqueous hydrochloric acid with a concentration of 1 mol / L to obtain solution b.
[0015] Add solution b to solution a, stir for 2 minutes after mixing, and let stand for 24 hours. Adjust the pH value to 7.0 with 1mol / L sodium hydroxide aqueous solution to obtain a flocculent precipitate, which is centrifuged, washed three times with deionized water, and dried at 100°C for 6 hours to obtain a blue-yellow solid, that is, poly-p-methoxyaniline-loaded nanoparticles palladium.
[0016] 2. Synthesis of biaryls:
[0017] In a 50 ml reaction tube, add 6 mg poly-p-methoxyaniline supported nano-palladium catalyst, 2.04 g (10 mmol) iodobenzene, 589 mg (10 mmol) hydrazine hydrate (85%), 1.29 g (10 mmol) di Isopropylethyla...
Embodiment 2
[0019] Other conditions are the same as in Example 1, and the influence of the reaction temperature on the reaction is checked, and the experimental results are as shown in Table 1.
[0020] Table 1 Effect of reaction temperature
[0021] Reaction temperature (°C) 80 130 140 150 Product yield (%) 7.3 52.3 70.3 53.3
[0022] It can be seen from the above table that the effect is the best and the yield is the highest when the reaction temperature is 140°C.
Embodiment 3
[0024] Other conditions are the same as in Example 1, and the influence of the consumption of the reducing agent hydrazine hydrate on the reaction is checked, and the experimental results are shown in Table 2.
[0025] Table 2 The effect of reducing agent hydrazine hydrate dosage
[0026] Hydrazine hydrate / mmol 0 0.5 1.0 2.0 Product yield (%) 41.0 39.8 70.3 14.0
[0027] It can be seen from the above table that the yield is the highest when the molar ratio of reducing agent hydrazine hydrate to iodobenzene is 1:1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com