9-phenyl-substituted fluorene derivative, and preparation method and application thereof
A technology of phenyl substitution and derivatives, which is applied in the field of organic compound synthesis, can solve the problems of few reports on orange and red phosphorescent materials, and achieve good electroluminescence efficiency, simple synthesis route, and excellent thermal stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
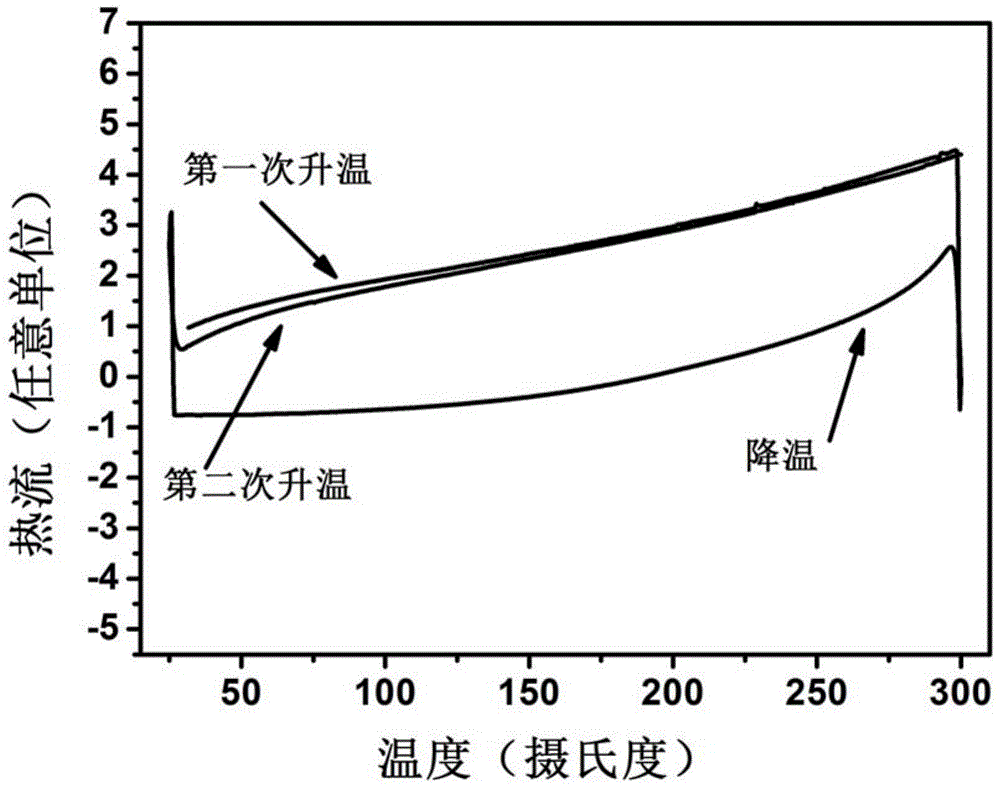
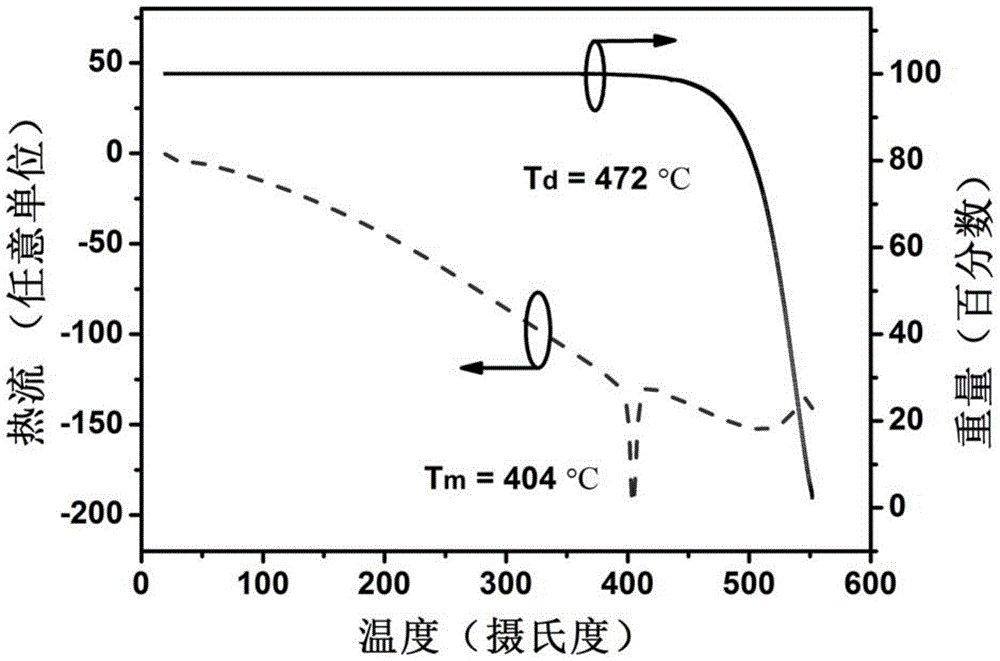
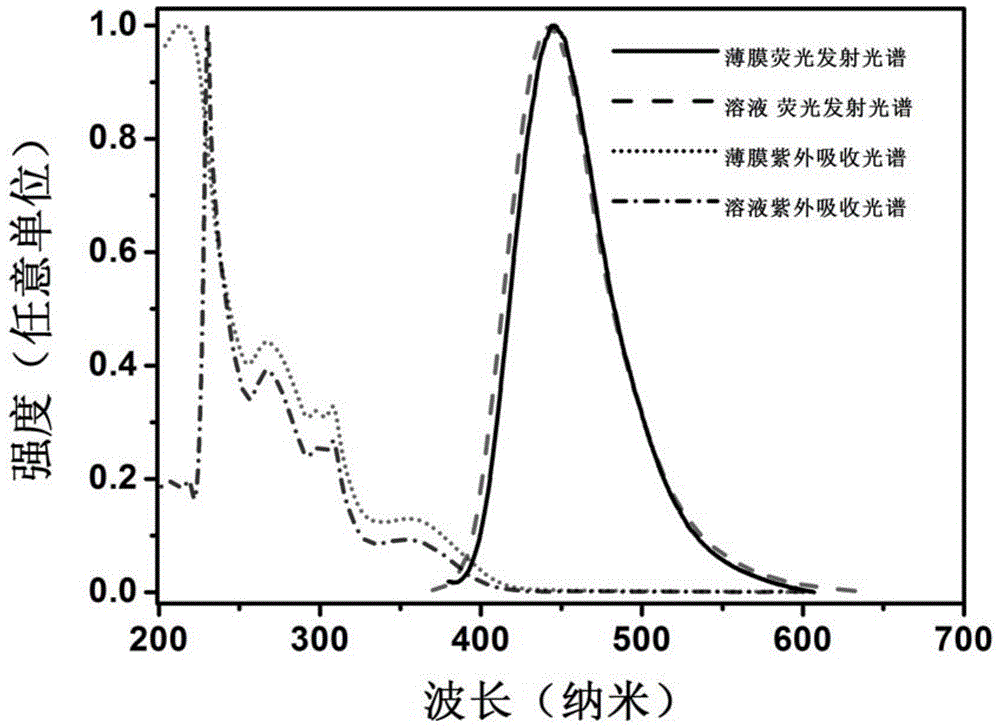
[0045] The present invention designs and synthesizes a class of blue-light host materials with high-efficiency fluorescent emission, whose basic framework is 1,4-bis(9-phenylfluorene)-phenylene, and naphthylaniline as a derivative of a functional substituent group. The molecular structure and configuration of this series of materials were characterized by mass spectrometry (EIMS or TOF), proton nuclear magnetic resonance and carbon nuclear magnetic resonance. The photophysical properties of the compounds were studied by UV absorption spectroscopy. The electrochemical properties of the compounds were studied by cyclic voltammetry. The thermodynamic properties of the compounds were studied by differential thermal-differential gravimetric analysis (DTA-TGA).
[0046] 1. The synthetic route is shown in Formula 1 below.
[0047] Synthesis of 1,4-bis(9-p-bromophenylfluorene)benzene (DBPFB).
[0048] The specific implementation method is as follows:
[0049] Under a nitrogen atmo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com