A class of benzothrone liquid crystal dyes containing a 3-position substituent, its preparation method and application
A technology of benzranthrone and a substituent is applied in the field of display materials, which can solve the problems that various characteristics of liquid crystal materials cannot be satisfied, and achieve the effects of novel structure, improved linear structure, and improved photoelectric performance.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
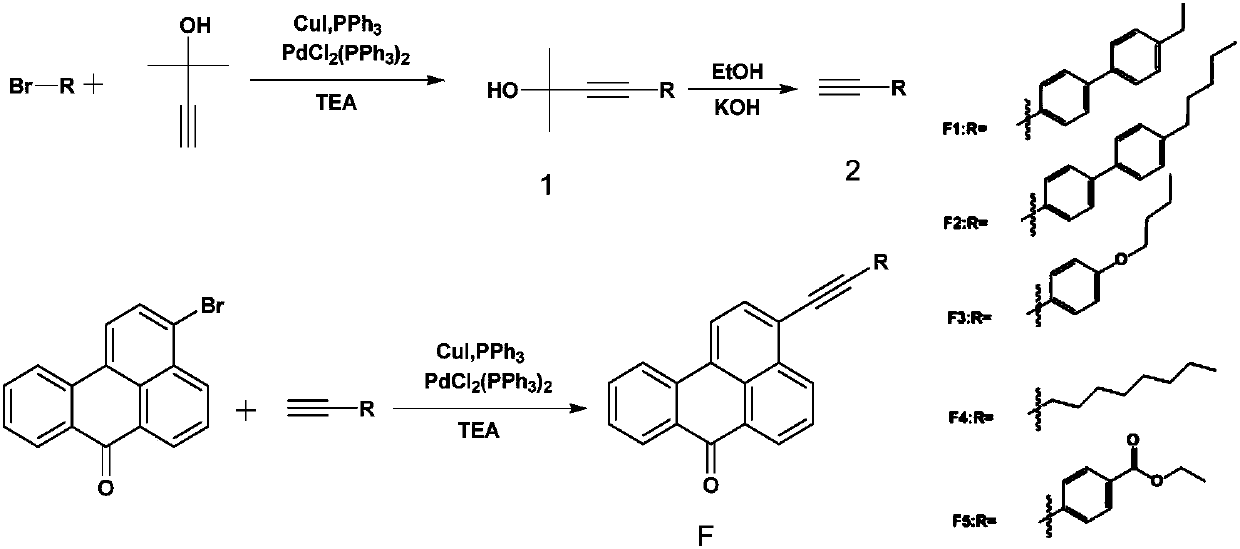
[0028] Synthesis of Liquid Crystal Dye F1
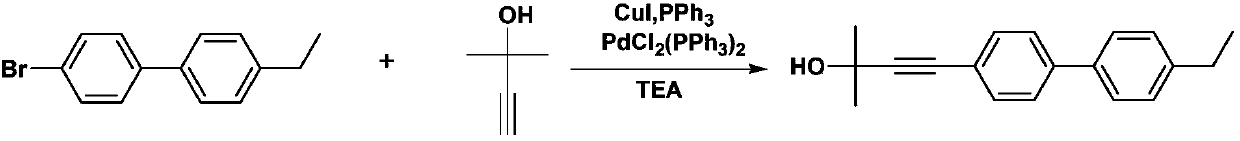
[0029] (1) Synthesis of 2-methyl-4-(4'-ethylbiphenyl) 3-butyne-2-methanol (compound 1)
[0030]
[0031] 302 mg (1 mmol) 4'-bromo-4-ethylbiphenyl and 100 μL (1 mmol) 2-methyl-3-butyn-2-ol and 7 mg ditriphenylphosphine palladium dichloride, 15 mg triphenyl Phosphine and 8 mg CuI were added as catalysts into a 50 mL double-necked round bottom flask, and 15 mL of dry triethylamine was used as an acid-binding agent and solvent. Stir under nitrogen protection and heat to reflux to complete the reaction. Triethylamine was removed under reduced pressure, the crude product was subjected to silica gel chromatography (eluent: dichloromethane), and the solvent was removed under reduced pressure to obtain 281.7 mg of a white solid with a yield of 92%. Melting point: 48.4-49.7°C.
[0032] 1 H-NMR (400MHz, CDCl3): δ=7.54(s, 4H), 7.50(d, J=8.0Hz, 2H), 7.25(d, J=3.2Hz, 2H), 3.12(s, 1H), 2.68 -2.61(m,2H),1.69-1.60(m,2H),1.38-1.31(m,4H),0.90(t...
Embodiment 2
[0044] Synthesis of liquid crystal dye F2
[0045] The synthetic method is the same as F1, and the 4'-bromo-4-ethylbiphenyl in step (1) is changed into 4'-bromo-4-n-pentylbiphenyl. The developer in step (3) was DCM:PE=2:1, and 406.3 mg of the final product was obtained as a yellow solid with a yield of 85%.
[0046] 1 H NMR (500MHz, CDCl3) δ8.84(dd, J=19.2, 7.7Hz, 2H), 8.51(d, J=7.9Hz, 1H), 8.43(d, J=7.8Hz, 1H), 8.33(d ,J=8.1Hz,1H),7.96–7.84(m,2H),7.75(dd,J=15.9,8.1Hz,3H),7.66(d,J=8.3Hz,2H),7.57(t,J= 8.3Hz, 3H), 7.29(d, J=8.1Hz, 2H), 2.70–2.63(m, 2H), 1.72–1.62(m, 2H), 1.42–1.32(m, 4H), 0.95–0.88(m ,3H).
[0047] 13 C NMR (126MHz, CDCl3) δ153.46(s), 144.11(s), 141.40–141.13(m), 137.51(s), 137.03(s), 136.10(s), 132.44(s), 132.26(s) ,131.43(s),130.23(s),129.71(s),129.61(d,J=5.4Hz),129.43(d,J=4.1Hz),128.76(s),128.47(s),128.13(s) ,128.13(s),126.96(s),125.10(s),123.67(s),122.52(s),122.30(d,J=5.7Hz),97.13(s),87.39(s),58.41(s) , 29.71(s), 28.56(s), 18.46(s), 15.56(s).
[004...
Embodiment 3
[0050] Synthesis of Liquid Crystal Dye F3
[0051] The synthetic method is the same as step (3) in F1, the difference is that the 4'-ethynyl-4-ethylbiphenyl in the step (3) is changed into p-n-butoxyphenylacetylene. The developer was DCM:PE=1:2, and 313.6 mg of the orange-yellow final product was obtained with a yield of 78%.
[0052] 1 H NMR (500MHz, CDCl3) δ8.82(dd, J=15.1, 7.7Hz, 2H), 8.50(d, J=7.9Hz, 1H), 8.41(d, J=7.7Hz, 1H), 8.33(d ,J=8.1Hz,1H),7.88(dd,J=16.2,8.0Hz,2H),7.75(t,J=7.6Hz,1H),7.64–7.52(m,3H),6.94(d,J= 8.7Hz, 2H), 4.02(t, J=6.5Hz, 2H), 1.84–1.76(m, 2H), 1.57–1.48(m, 2H), 1.00(t, J=7.4Hz, 3H).
[0053] 13 C NMR (126MHz, CDCl3) δ183.61(s), 159.79(s), 135.75(s), 133.47(s), 133.35(s), 133.26(s), 132.71(s), 130.30(s), 130.12 (s), 128.41(s), 128.16(s), 126.99(s), 126.56(s), 123.86(s), 123.52(s), 123.15(s), 114.70(d, J=9.7Hz), 97.48 (s), 85.90(s), 67.87(s), 31.26(s), 19.25(s), 13.86(s).
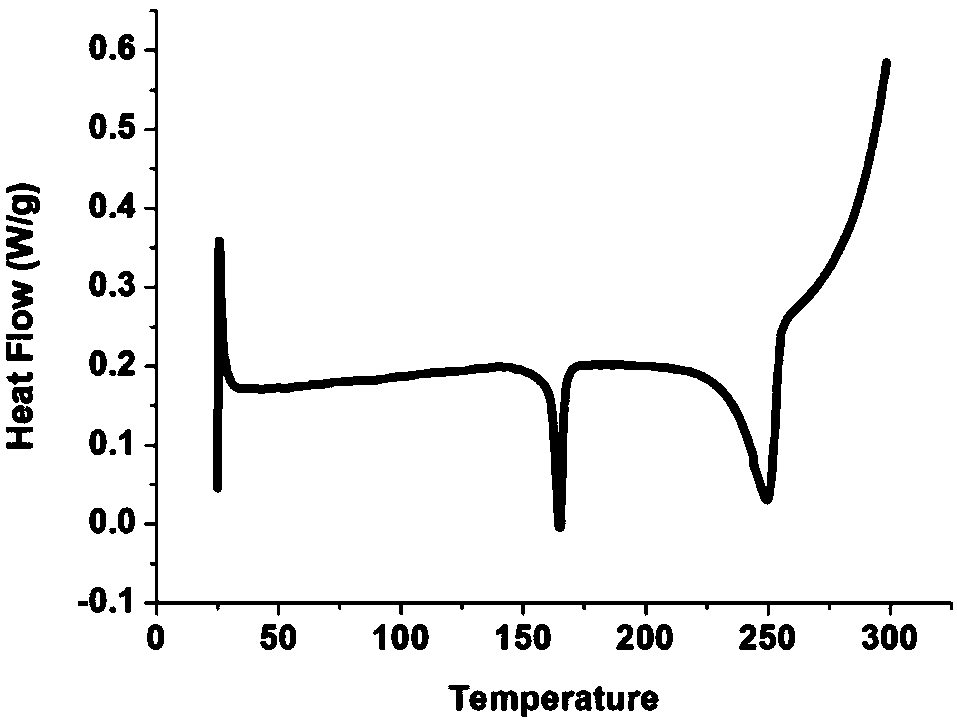
[0054] Melting point: 150.1-151.2°C. TOF MS EI+(m / z): Calculated value:...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com