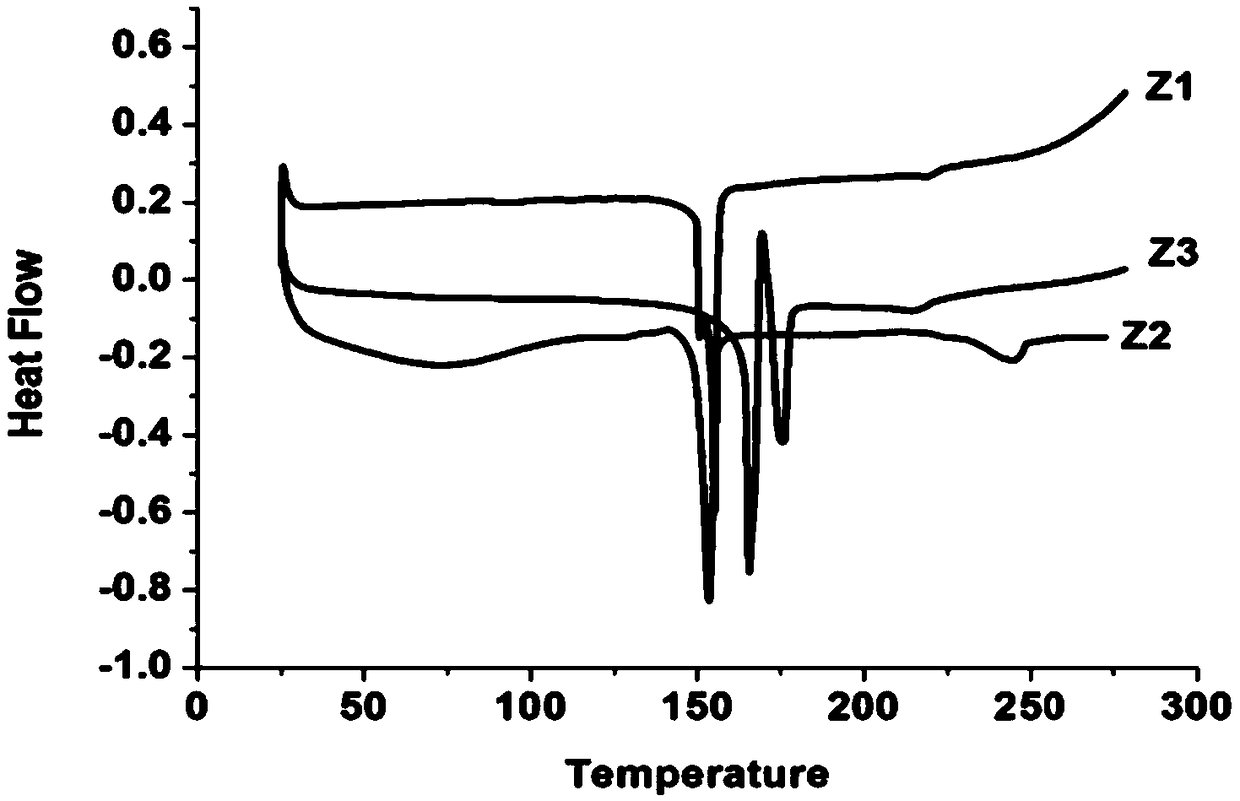
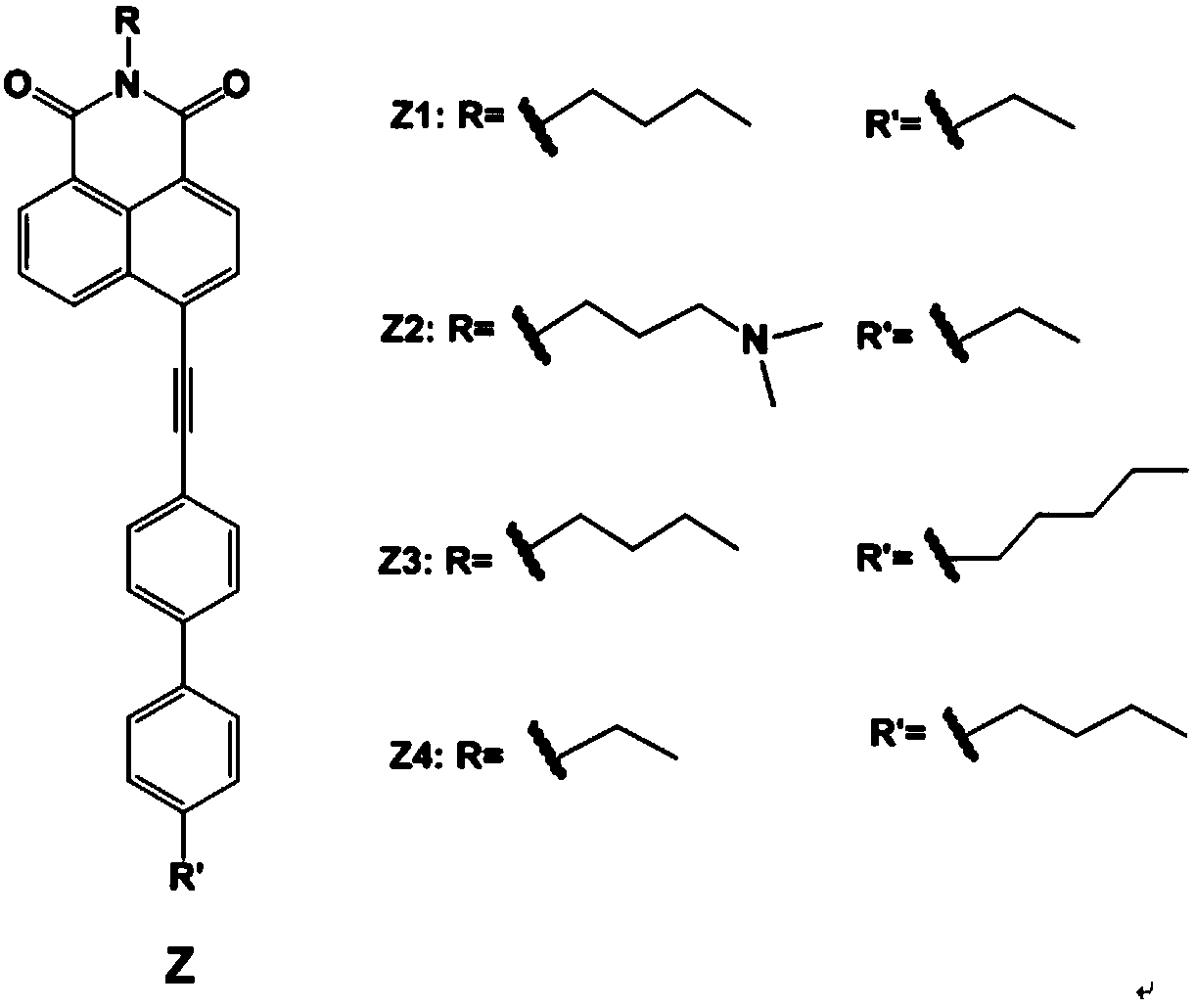
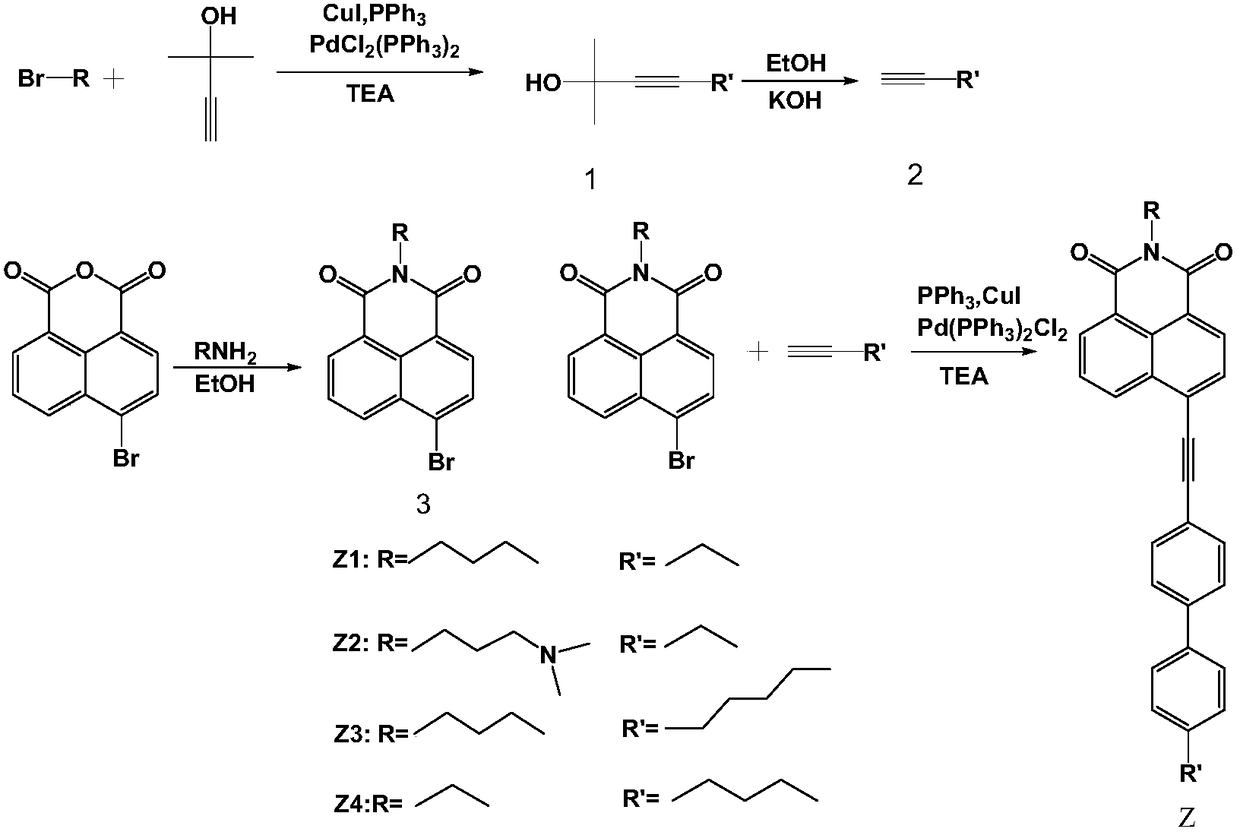
Liquid crystal compound containing 4-(biphenylethynyl)-1,8 naphthalimide, preparation method and application thereof
A technology of naphthalimide and biphenylacetylene, which is applied in the field of display materials, can solve the problems of not being able to meet the various photoelectric characteristics of liquid crystal displays, and achieve the effects of novel structure, good order parameters, and easy-to-obtain raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Synthesis of liquid crystal dye Z1
[0031] (1) Synthesis of 2-methyl-4-(4'-ethylbiphenyl) 3-butyne-2-methanol (compound 1)
[0032]
[0033] 302 mg (1 mmol) 4'-bromo-4-ethylbiphenyl and 100 μL (1 mmol) 2-methyl-3-butyn-2-ol and 7 mg ditriphenylphosphine palladium dichloride, 15 mg triphenyl Phosphine and 8 mg CuI were added as catalysts into a 50 mL double-necked round bottom flask, and 15 mL of dry triethylamine was used as an acid-binding agent and solvent. Stir under nitrogen protection and heat to reflux to complete the reaction. Triethylamine was removed under reduced pressure, the crude product was subjected to silica gel chromatography (eluent: dichloromethane), and the solvent was removed under reduced pressure to obtain 281.7 mg of white solid 2-methyl-4-(4'-ethylbiphenyl) 3 - Butyne-2-methanol, yield 92%. Melting point: 48.4-49.7°C.
[0034] 1 H-NMR (400MHz, CDCl3): δ=7.54(s, 4H), 7.50(d, J=8.0Hz, 2H), 7.25(d, J=3.2Hz, 2H), 3.12(s, 1H), 2.68 -2.61(m,...
Embodiment 2
[0048] Synthesis of liquid crystal dye Z2
[0049] In addition to replacing n-butylamine with 3-dimethylaminopropylamine in step (3), replace it with 4-bromo-N-(3-dimethylamino-n-propyl)-1,8-naphthalimide in step (4). Except for 4-bromo-N-n-butyl-1,8-naphthoimide, other operating steps were the same as in Example 1 to obtain bright yellow solid Z2 with a yield of 60%.
[0050] 1 H NMR (500MHz, CDCl 3 )δ8.77(dd, J=8.4,1.1Hz,1H),8.65(dd,J=7.3,1.1Hz,1H),8.56(d,J=7.6Hz,1H),7.97(d,J=7.6 Hz,1H),7.85(dd,J=8.3,7.3Hz,1H),7.76–7.71(m,2H),7.70–7.64(m,2H),7.57(d,J=8.2Hz,2H),7.32 (d, J=8.3Hz, 2H), 4.28(t, J=7.3Hz, 2H), 2.77–2.65(m, 4H), 2.46(s, 6H), 2.08(dt, J=14.2, 7.2Hz, 2H), 1.29(t, J=7.6Hz, 3H).
[0051] 13 C NMR (126MHz, CDCl 3 )δ163.87(d,J=34.5Hz),144.30(s),142.20(s),137.33(s),132.57(s),132.35(s),131.69(s),131.57(s),130.49( s), 130.49(s), 128.51(s), 128.05(s), 127.91(s), 127.44(s), 127.07(s), 126.99(s), 122.74(s), 121.73(s), 120.63( s), 99.48(s), 86.82(s), 56.73(s), 53.43(s)...
Embodiment 3
[0054] Synthesis of liquid crystal dye Z3
[0055] In addition to replacing 4'-bromo-4-ethylbiphenyl with 4'-bromo-4-n-pentylbiphenyl in step (1), 2-methyl-4-(4'- n-pentylbiphenyl) 3-butyne-2-methanol is replaced with 2-methyl-4-(4'-ethylbiphenyl) 3-butyne-2-methanol, and other operating steps are the same as in Example 1, to obtain Yellow solid Z3, 70% yield.
[0056] 1 H NMR (500MHz, CDCl 3 )δ8.76(dd, J=8.3,0.9Hz,1H),8.67–8.63(m,1H),8.57(d,J=7.6Hz,1H),7.97(d,J=7.6Hz,1H), 7.85(dd,J=8.2,7.4Hz,1H),7.76–7.71(m,2H),7.69–7.64(m,2H),7.56(d,J=8.2Hz,2H),7.29(d,J= 8.2Hz, 2H), 4.19(dd, J=8.5, 6.7Hz, 2H), 2.71–2.62(m, 2H), 1.74(ddd, J=15.3, 11.0, 7.6Hz, 2H), 1.66(dd, J =15.1,7.5Hz,2H),1.46(dq,J=14.9,7.4Hz,2H),1.40–1.33(m,4H),1.01–0.97(m,3H),0.94–0.88(m,3H).
[0057] 13 C NMR (126MHz, CDCl 3 )δ163.86(d,J=33.7Hz),143.00(s),142.15(s),137.30(s),132.33(s),131.54(s),130.66(s),130.34(s),129.04( s), 128.05(s), 127.62(s), 127.39(s), 127.05(s), 126.89(s), 123.02–122.82(m), 122.07(s), 120...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com