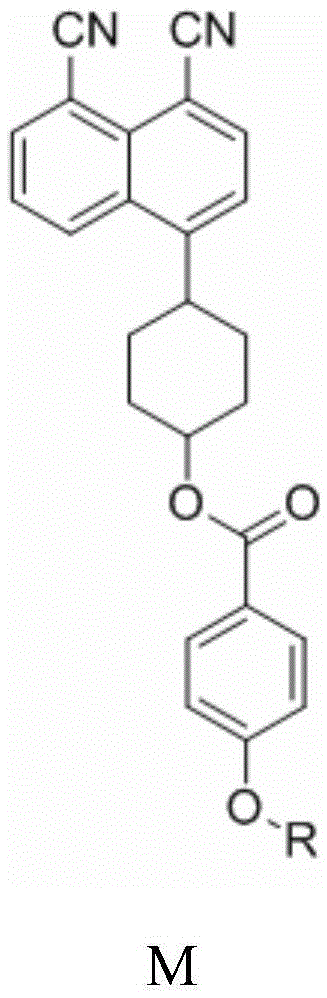
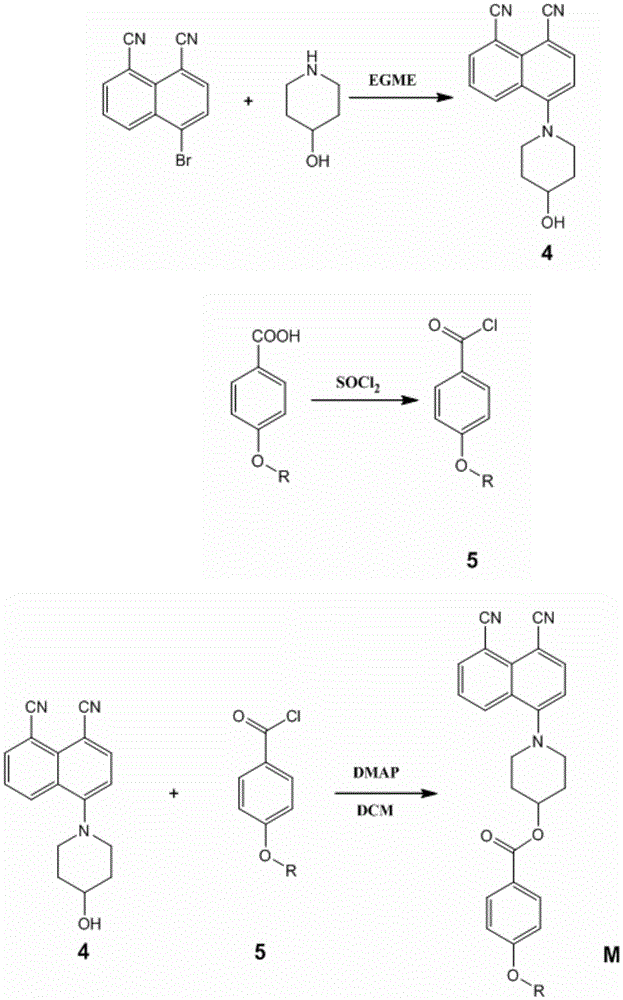
A class of fluorescent dichroic dyes containing 4-(p-hydroxypiperidinyl)-1,8-naphthalene dinitrile, its preparation method and application
A dichroic dye and fluorescence technology, which is applied in the field of display materials, can solve the problems of dichroic dyes with many structures and varieties, and limited application performance, and achieve the effects of novel structure, easy-to-obtain raw materials, and improved solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Synthesis of Fluorescent Dichroic Dye M1
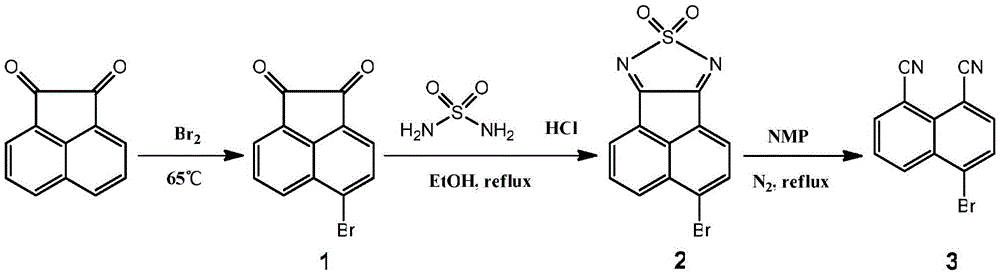
[0030] (1) Synthesis of 4-bromoacenaphthylquinone (compound 1)
[0031]
[0032] Add 10.02g (55mmol) of acenaphthoquinone into a 250mL three-neck flask, then measure 12mL of liquid bromine with a syringe and add it into it. Saturated NaOH solution absorbs the tail gas, slowly heats to 65°C, and reacts for two hours under stirring. The reaction was stopped, the mixture was cooled to room temperature, and an appropriate amount of saturated Na 2 SO 3 The solution was freed of excess bromine and hydrogen bromide until the liquid was colorless. Suction filtration and washing until the filtrate is neutral. The crude product was recrystallized with glacial acetic acid after drying to obtain 12.34 g of a yellow solid with a yield of 86%. Melting point: 236.0-236.8°C.
[0033] (2) Synthesis of 3-bromo-8-thia-7,9-diaza-cyclopenta[a]acenaphthene 8,8-dioxide (compound 2)
[0034]
[0035] Add 11.49g (45mmol) of 4-bromoacenaphth...
Embodiment 2
[0051] Synthesis of Fluorescent Dichroic Dye M2
[0052] Except in step (5) with p-butoxybenzoic acid instead of p-methoxybenzoic acid reaction to obtain p-butoxybenzoyl chloride, in step (6) react with p-butoxybenzoyl chloride and compound 4 to obtain M2 Except, other operating steps are the same as in Example 1, and the yield of the target product M2 is 80%, melting point: 195.7-196.2°C.
[0053] 1 H-NMR (400MHz, CDCl 3 ):δ=8.48(dd, J=8.4,0.8Hz,1H),8.10(dd,J=7.2,0.8Hz,1H),8.02(dd,J=8.4,5.2Hz,3H),7.64(dd, J=8.4,7.2Hz,1H),7.21(d,J=8.0Hz,1H),6.93(d,J=8.8Hz,2H),5.30(s,1H),4.03(t,J=6.4Hz, 2H),3.41(s,2H),3.17(s,2H),2.29(s,2H),2.15(s,2H),1.85-1.74(m,2H),1.49(m,2H),0.99(t ,J=7.6Hz,3H).
[0054] ESIQ1MS: Calculated value: [C 28 h 27 N 3 o 3 ] + (m / z)=453.2052, measured value: [C 28 h 27 N 3 o 3 ] + (m / z) = 453.2048.
Embodiment 3
[0056] Synthesis of Fluorescent Dichroic Dye M3
[0057] Except in step (5) with p-hexyloxybenzoic acid instead of p-methoxybenzoic acid reaction to obtain p-hexyloxybenzoyl chloride, in step (6) react with p-hexyloxybenzoyl chloride and compound 4 to obtain M3 Except, other operating steps are the same as in Example 1, the yield of the target product M3 is 77%, and the melting point is 198.6-200.3°C.
[0058] 1 H-NMR (400MHz, CDCl 3 ):δ=8.48(d, J=8.4Hz, 1H), 8.10(d, J=7.2Hz, 1H), 8.02(dd, J=8.4, 5.3Hz, 3H), 7.68-7.60(m, 1H) ,7.21(d,J=8.0Hz,1H),6.93(d,J=8.8Hz,2H),5.30(s,1H),4.02(t,J=6.8Hz,2H),3.41(s,2H) ,3.17(s,2H),2.29(s,2H),2.16(s,2H),1.86-1.76(m,2H),1.47(dt,J=14.4,7.2Hz,2H),1.35(td,J =6.8,3.2Hz,4H),0.91(t,J=6.8Hz,3H). 13 C-NMR (126MHz, CDCl 3 ):δ=165.60,163.15,155.69,138.54,137.65,131.61,130.45,130.26,128.62,125.66,122.34,117.48,117.01,115.39,114.15,109.03,101.93,68.88,68.29,50.59,31.56,31.14,29.72, 29.07, 25.67, 22.61, 14.08.
[0059] TOFMSEI+: Calculated value: [C...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com