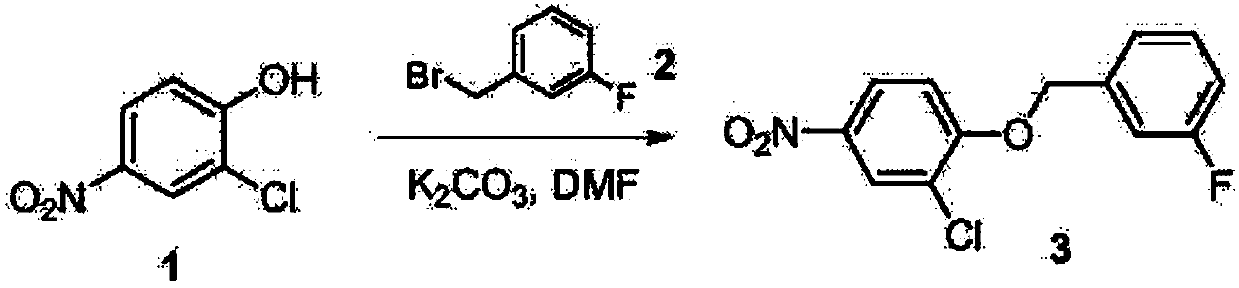
A kind of synthetic method of 3-chloro-4-(3-fluorobenzyloxy) nitrobenzene
A technique for the synthesis of fluorobenzyloxy, which is applied in the field of synthesis of 3-chloro-4-nitrobenzene, can solve problems such as low yield, high irritation of m-fluorobenzyl bromide, and great harm to human body, and achieve the product High yield, easy industrial production, and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
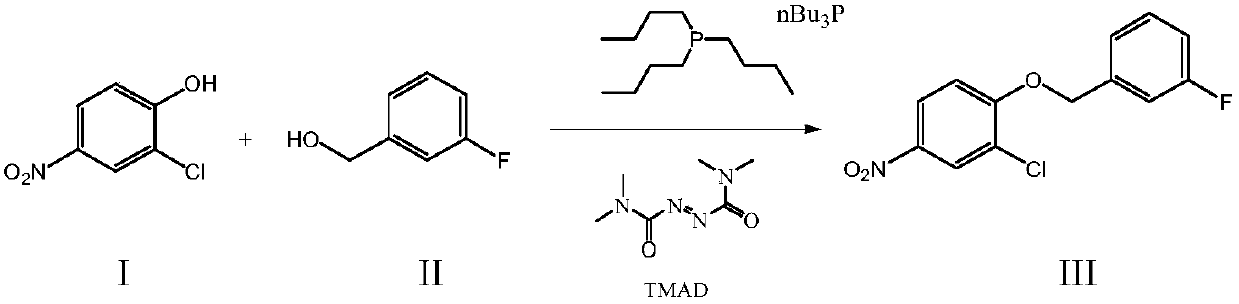
[0018] Add 350ml tetrahydrofuran, 75g 2-chloro-4-nitrophenol, 57.2g 3-fluorobenzyl alcohol and 104.9g tributylphosphine into a 1000ml reaction flask, start stirring, cool down to 10°C, and slowly add 89.3g tetramethyl A mixed solution composed of azodicarbonamide (TMAD) and 200ml tetrahydrofuran, after the dropwise addition is completed, the temperature is controlled at 10°C and the reaction is stirred for 2 hours. 200ml of dimethylformamide was stirred and dissolved, cooled to 0°C for recrystallization, suction filtered, and the obtained solid was dried at 55-60°C to obtain 112.7g of light yellow solid with a purity of 99.89% and a yield of 92.6%.
Embodiment 2
[0020] Add 350ml tetrahydrofuran, 75g 2-chloro-4-nitrophenol, 58.0g 3-fluorobenzyl alcohol and 106g tributylphosphine into a 1000ml reaction flask, start stirring, cool down to 10°C, and slowly add 90g tetramethylazo A mixed solution composed of dimethylamide (TMAD) and 200ml tetrahydrofuran, the dropwise addition is completed, the temperature is controlled at 10°C and the reaction is stirred for 1.5 hours. 200ml of methylformamide was stirred and dissolved, cooled to 0°C for recrystallization, filtered with suction, and the resulting solid was dried at 55-60°C to obtain 113.2g of a light yellow solid with a purity of 99.82% and a yield of 93.0%.
Embodiment 3
[0022] Add 350ml tetrahydrofuran, 75g 2-chloro-4-nitrophenol, 57.2g 3-fluorobenzyl alcohol and 100.0g tributylphosphine to a 1000ml reaction flask, start stirring, cool down to 10°C, and slowly add 85.0g tetramethyl A mixed solution composed of azodicarbonamide (TMAD) and 180ml tetrahydrofuran, the dropwise addition is completed, the temperature is controlled at 10°C and the reaction is stirred for 3 hours. - 200ml of dimethylformamide was stirred and dissolved, cooled to 0°C for recrystallization, filtered with suction, and the obtained solid was dried at 55-60°C to obtain 112.5g of a light yellow solid with a purity of 99.93% and a yield of 92.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com