Expression vector, expression system, preparation method and application of mammal cell attachment on basis of cHS4 element
An expression vector and mammalian technology, applied in the field of genetic engineering and gene therapy, can solve the problems of transgene silencing, low copy number, poor vector stability, etc., achieve lasting expression, good safety, and overcome the effect of transgene silencing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
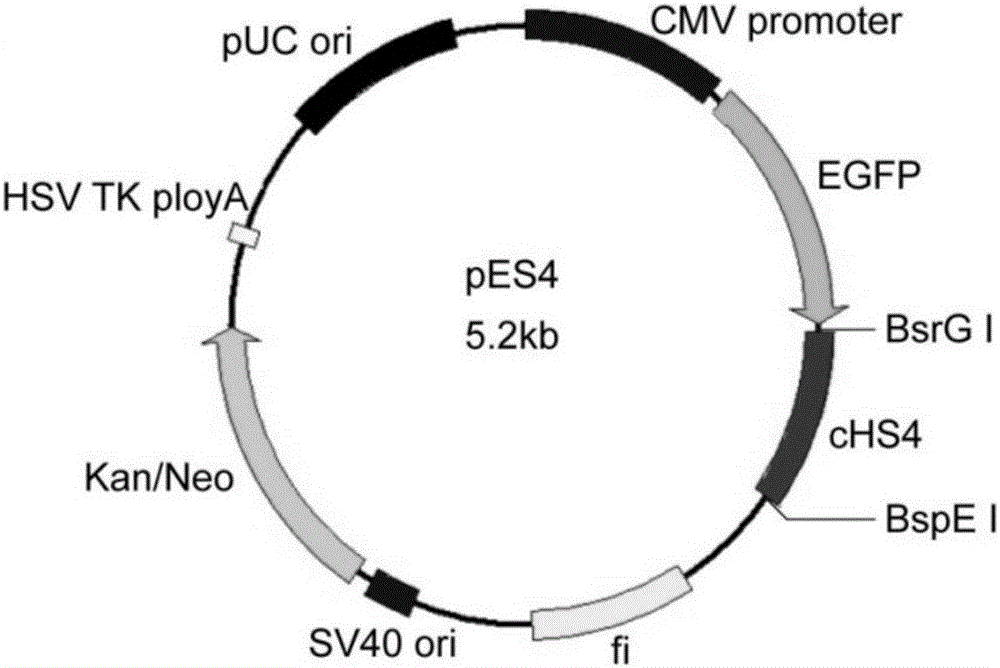
[0037] The construction of the episomal expression vector pES4, the steps are as follows:
[0038] 1) Synthesis of cHS4 sequence
[0039] The cHS4 sequence was synthesized according to the literature (Saunders F, Sweeney B, Antoniou, et al. Chromatin function modifying elements in an industrial antibody production platform-comparison of UCOE, MAR, STAR and cHS4 elements.2015, PLoS One 10:e0120096.) for directional cloning And the identification of subsequent vectors, the restriction sites of BspE I and BsrG I were respectively introduced at the 5' end of the sequence. Since the pES4 vector was constructed using seamless cloning technology, the company added 15 bp homologous sequences to both ends of the sequence when synthesizing the cHS4 sequence. The synthetic cHS4 sequence is as follows:
[0040]
[0041] Wherein, the parts in italics represent homologous sequences, and the underlined parts are enzyme cutting sites.
[0042] 2) Construction of pES4 plasmid vector
[...
Embodiment 2
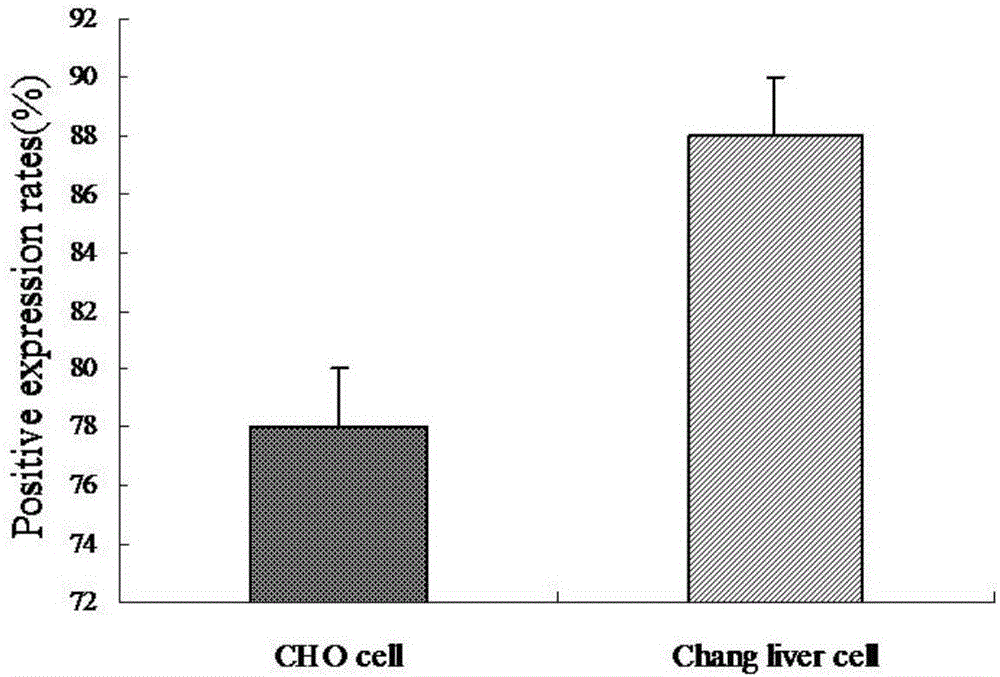
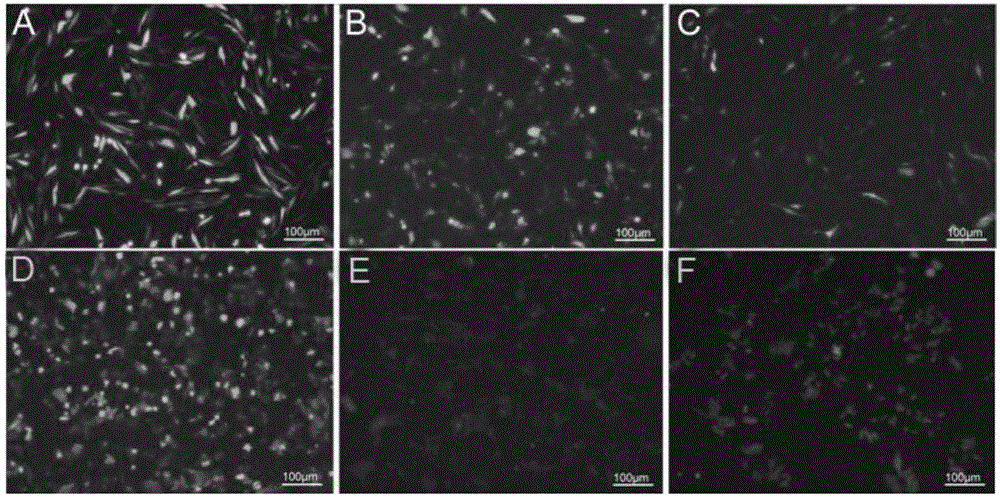
[0051] The construction of mammalian cell expression system, the steps are as follows:
[0052] 1) Synthesis of IFN-β sequence
[0053] According to the IFN-β sequence published by NCBI (GenBank: BC096151.2, bases 1 to 564), the company synthesized IFN-β (as shown in SEQ ID NO: 3, with 15 bp vectors added to both ends of the sequence) Homologous sequence, and IFN-β is inserted downstream of eGFP), which was completely consistent with the sequence registered in GenBank by sequencing analysis.
[0054] 2) Construction of the episomal expression vector pES4-IFN-β containing the IFN-β sequence
[0055] Digest the pES4 plasmid with BsrG I. The enzyme digestion system is: 1 μg / μL pES4 plasmid 10 μL, 10×NE buffer 2 μL, BsrG I enzyme 0.5 μL, add triple distilled water to 20 μL; mix well, and digest in a 37°C water bath 8h. After the enzyme digestion, 1.5% agarose gel electrophoresis was performed to recover the digested pES4 linear plasmid DNA.
[0056] 10 μL In-Fusion Cloning lig...
PUM
| Property | Measurement | Unit |
|---|---|---|
| fluorescence | aaaaa | aaaaa |
| fluorescence | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com