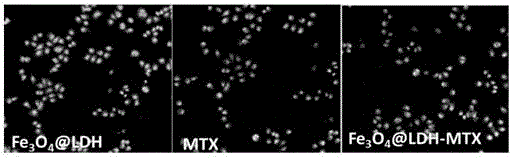
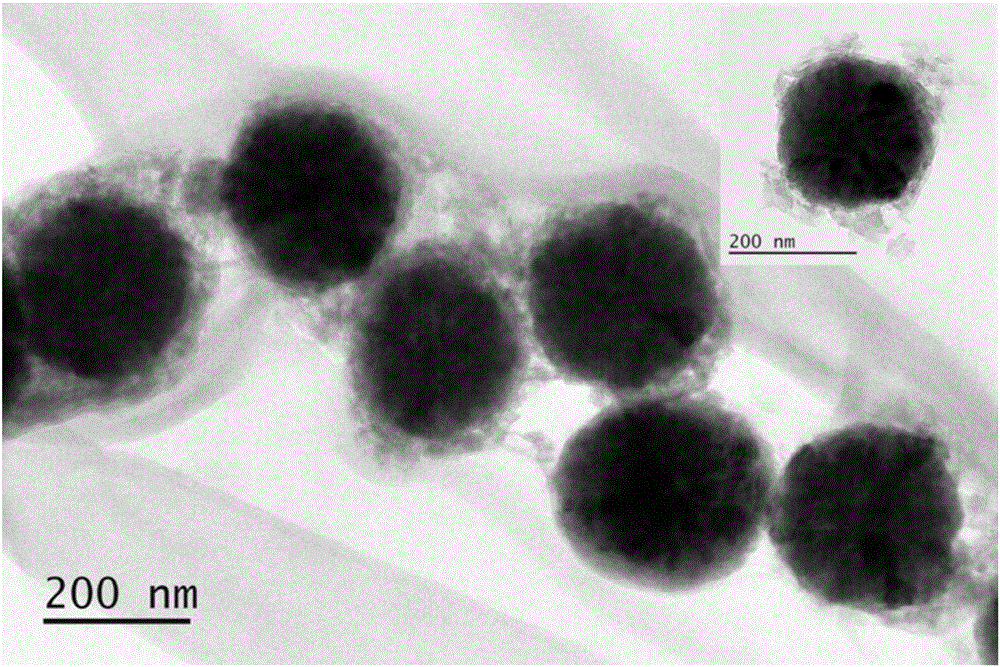
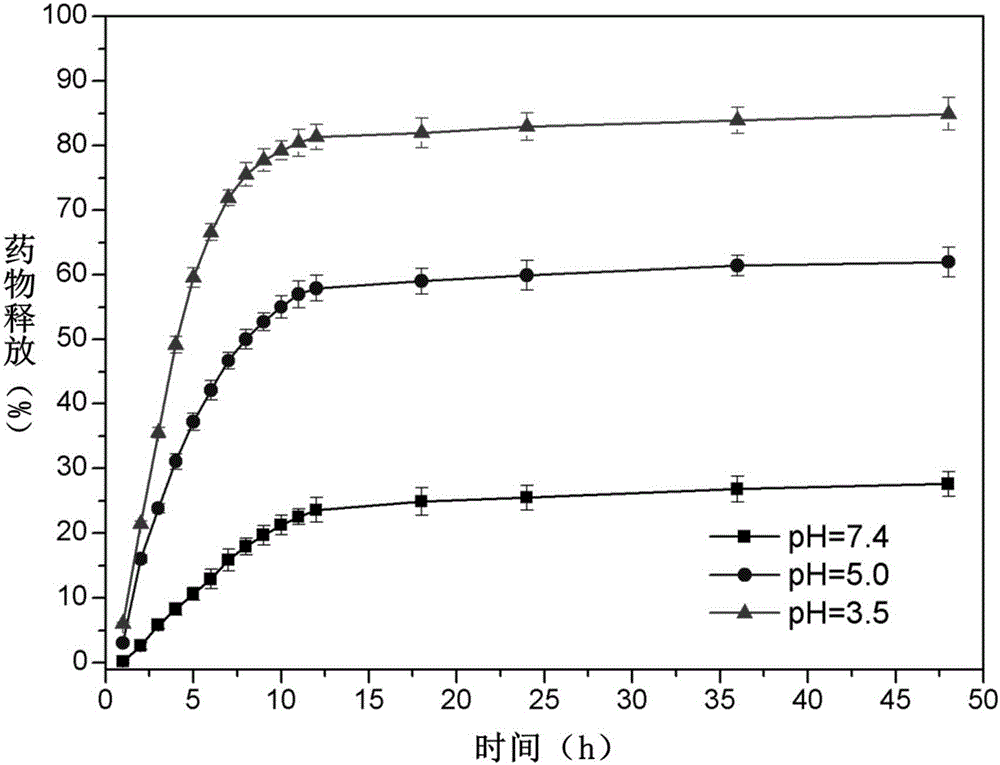
pH sensitive-type Fe3O4 coated LDH methotrexate-loaded nano drug particles, preparation method and application thereof
A technology of methotrexate and nano-drugs, which is applied in the direction of drug combinations, pharmaceutical formulas, medical preparations of non-active ingredients, etc., and can solve the problems of ineffective action on lesion sites, lack of identification of lesion sites, poor actual targeting performance, etc. problems, to achieve the effect of improving curative effect and bioavailability, good water solubility, and increasing drug loading
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Example 1: Fe 3 o 4 Preparation of @LDH-MTX
[0032] Sequentially weigh 0.5g FeCl 3 ·6H 2 O, 3.0g sodium acetate, dissolved in 40mL ethylene glycol, ultrasonically stirred for 20min to disperse the system evenly, then added 2.0g LDH to the mixed solution, continued ultrasonically stirred for 10min, then transferred the uniform mixed solution into polytetrafluoroethylene In the reaction kettle, react at 200°C for 10h. After the reaction was completed, the product was poured out, extracted by magnetic decantation, and washed several times with deionized water. The washed product was dried in a vacuum oven at 25°C to obtain dry powdered Fe 3 o 4 @LDH Carrier.
[0033] Sequentially weigh 50 mg of dry Fe 3 o 4 @LDH, 2.5mg MTX, dissolved in 20mL deionized water, ultrasonicated for 5min to make the system uniformly dispersed. Then transfer to a one-necked flask, and react in the dark at 55°C for 3 days.
[0034]After the reaction was completed, the product was poure...
Embodiment 2
[0037] Example 2: Fe 3 o 4 Preparation of @LDH-MTX
[0038] Sequentially weigh 0.5g FeCl 3 ·6H 2 O. Dissolve 3.0 g of sodium acetate in 40 mL of ethylene glycol, and stir ultrasonically for 20 min to disperse the system evenly, then add 2.0 g of LDH to the mixture, and continue ultrasonically stirring for 10 min. Afterwards, the homogeneous mixed liquid was transferred into a polytetrafluoroethylene reactor and reacted at 200°C for 8 hours. After the reaction was completed, the product was poured out, extracted by magnetic decantation, and washed several times with deionized water. Then, the product was dried in a vacuum oven at 25°C to obtain dry powdered Fe 3 o 4 @LDH Carrier.
[0039] Sequentially weigh 50 mg of dry Fe 3 o 4 @LDH, 3mg MTX, dissolved in 20mL deionized water, ultrasonicated for 5min to disperse the system evenly. Then transfer to a one-necked flask, and react in the dark at 55°C for 3 days.
[0040] After the reaction was completed, the product was...
Embodiment 3
[0041] Example 3: Fe 3 o 4 Preparation of @LDH-MTX
[0042] Sequentially weigh 0.5g FeCl 3 ·6H 2 O. Dissolve 3.0 g of sodium acetate in 40 mL of ethylene glycol, and stir ultrasonically for 20 min to disperse the system evenly. Then, 2.0 g LDH was added to the mixture, and ultrasonic stirring was continued for 10 min. Afterwards, the homogeneous mixed liquid was transferred into a polytetrafluoroethylene reactor, and reacted at 190° C. for 10 h. After the reaction was completed, the product was poured out, extracted by magnetic decantation, and washed several times with deionized water. Then, the product was dried in a vacuum oven at 30°C to obtain dry powdered Fe 3 o 4 @LDH Carrier.
[0043] Sequentially weigh 50 mg of dry Fe 3 o 4 @LDH, 5mg MTX, dissolved in 20mL deionized water, ultrasonic 5min to make the system evenly dispersed. Afterwards, it was transferred to a one-necked flask, and reacted in the dark at 65° C. for 3 days.
[0044] After the reaction was c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com