Isoprene genetic engineering producing strain and application thereof
A technology of genetically engineered bacteria and isoprene, applied in the field of bioengineering, can solve the problems of inability to produce large-scale industrialized isoprene, instability, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0075] The method for preparing isoprene of the present invention comprises: cultivating the isoprene genetically engineered bacteria to obtain isoprene.
[0076] The present inventors have achieved high efficiency in the application of prokaryotic cells by introducing an exogenous mevalonate pathway into host cells and overexpressing isoprene synthase, more preferably also enhancing the phosphomethylerythritol pathway in host cells. Production of isoprene.
[0077] Utilizing the method of the invention, the output of isoprene can reach more than 24g / L, and the conversion rate of carbon source exceeds 22%, which is at a very high level.
Embodiment 1
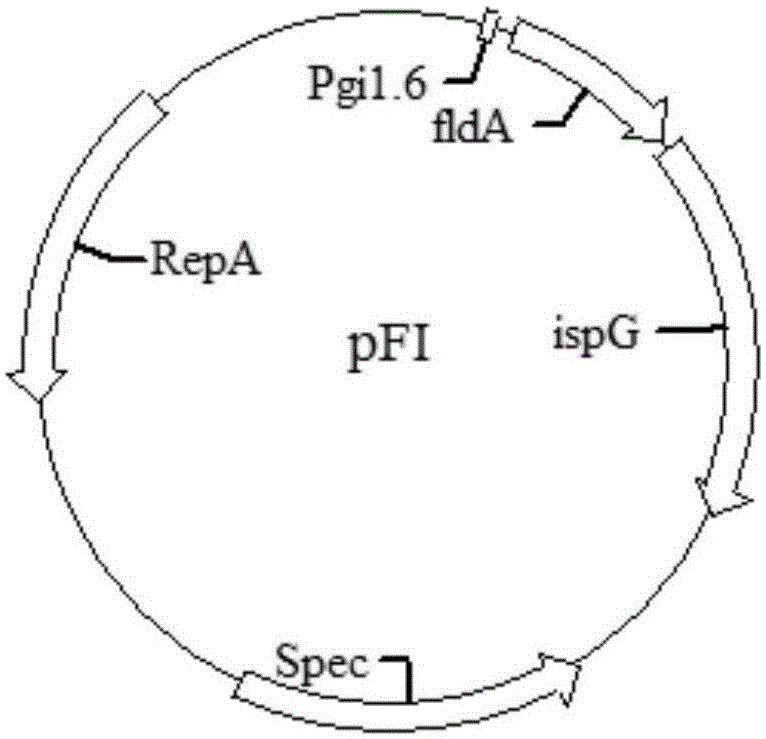
[0079] Example 1 : Construction of recombinant plasmid pFI
[0080] Using GI1.6-fldA-F / NruI-SacII-fldA-R and SacII-ispG-rbs-F / NruI-ispG-R as primers and E. coli genome as template, respectively, PCR amplification obtained GI1.6-fldA and ispG.
[0081] The GI1.6-fldA fragment was digested with SalI and cloned into the SalI / SamI site of pCL1920 vector (obtained from Shandong University), the ligated product was transferred into DH5α competent cells, and spread on a plate containing spectinomycin resistance for screening culture , the positive plasmid can be verified by EcoRI digestion, and a fragment of about 1kb / 4.3kb should be obtained to obtain pCL-fldA.
[0082]The ispG fragment was cloned into the SacII / NruI site of pCL-fldA by SacII digestion, the ligation product was transferred into DH5α competent cells, spread on a plate containing spectinomycin resistance for screening and culture, and the positive plasmid was verified by EcoRI digestion. A fragment of about 2.0kb / ...
Embodiment 2
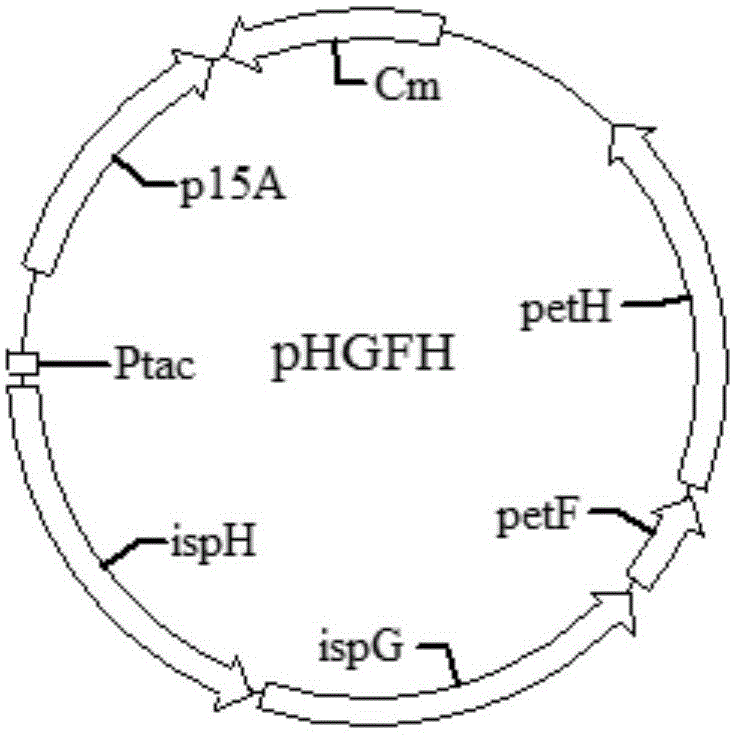
[0091] Example 2 : Construction of recombinant plasmid pHGFH
[0092] ispH is derived from Anabaena sp.PCC7120 (GenBank accession number: NC_003272.1), ispG, petF, petH are derived from T.elongatus BP-1 (GenBank accession number: NC_004113.1).
[0093] See GenBank accession number for ispH sequence: GI:499304384;
[0094] See the GenBank accession number for the ispG sequence: GI: 22298539;
[0095] See the GenBank accession number for the petF sequence: GI: 22298552;
[0096] See GenBank accession number: GI:22298754 for the sequence of petH.
[0097] The above four genes ispH, ispG, petF, petH were codon optimized for expression. The expression was initiated by the Ptac promoter (GenBank accession number: E02927), the terminator sequence was derived from aspA (GenBank accession number: CP001164), and each fragment was ligated to form the Ptac-ispH-ispG-petF-petH-term sequence.
[0098] Artificially synthesized Ptac-ispH-ispG-petF-petH-term sequence, and added SmaI rest...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com