Synthesis method for pharmaceutically acceptable salt of alkylhydrazine
A synthesis method and technology of alkyl hydrazine, which are applied in hydrazine preparation, organic chemistry and other directions, can solve the problems of high industrial scale-up cost, difficult scale-up production, large amount of waste water, etc., and achieve the effects of low cost, short synthesis route and low price.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
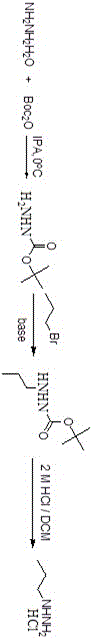
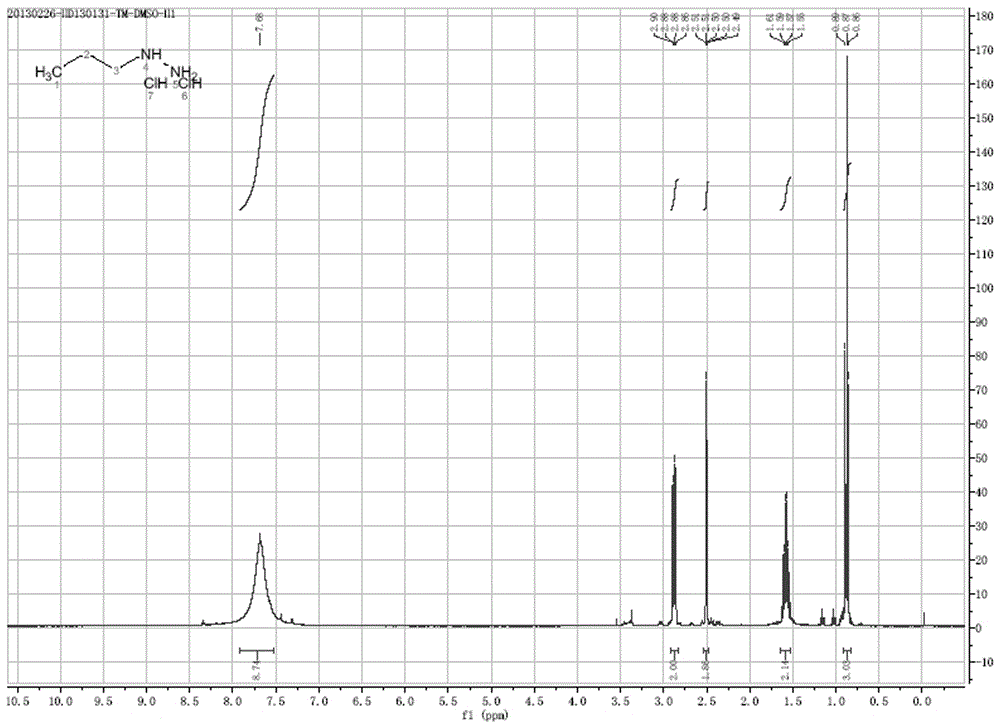
[0035] Example 1 Synthesis of n-propylhydrazine hydrochloride, see the synthetic route figure 1 , Including the following steps:
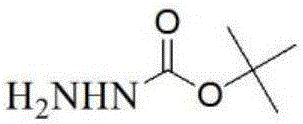
[0036] (1) Synthesis of BOC hydrazine: dissolve the raw material hydrazine hydrate in the isopropanol (IPA) solvent, the volume-weight ratio (ml / g) of isopropanol to the hydrazine hydrate is 5-20:1, and then add Add BOC anhydride to the solution, the molar ratio of hydrazine hydrate and BOC anhydride is 1~1.5:1~2:1, stir at 0℃ until the reaction is complete (about 0.5h), concentrate under reduced pressure until the solvent is clear, dichloromethane Dilute, dry with anhydrous sodium sulfate, and concentrate under reduced pressure to obtain a white solid. The white solid is slurried with petroleum ether, and the volume-weight ratio of the petroleum ether to the white solid is 5-10:1, and then the BOC hydrazine is obtained by vacuum filtration with a yield: 50%;
[0037] (2) Synthesis of BOC n-propyl hydrazine: Add the BOC hydrazine obtained in step (1) a...
Embodiment 2
[0039] Example 2: Synthesis of BOC Hydrazine:
[0040] Add 30 g of hydrazine hydrate and 150 ml of isopropanol to a three-necked reaction flask with a stirrer, add 60 ml of an isopropanol solution of 55.8 g of BOC anhydride under ice-water bath cooling, and stir for 0.5h at 0-10°C until the reaction is complete , Concentrate until the solution is clear, add 100 ml of dichloromethane, dry with anhydrous sodium sulfate, and concentrate under reduced pressure to obtain a white solid. The obtained crude product was slurried with 200 ml of petroleum ether and filtered under reduced pressure to obtain 35.7 g of white solid, with an LC purity of over 92% and a yield of 50%.
Embodiment 3
[0041] Example 3: Synthesis of BOC Hydrazine
[0042] Add 30 g of hydrazine hydrate and 150 ml of isopropanol to a three-necked reaction flask with a stirrer, add 120 ml of isopropanol solution of 111.6 g of BOC anhydride under ice-water bath cooling, and stir at 0-10℃ for 0.5h until the reaction is complete , Concentrate until the solution is clear, add 100 ml of dichloromethane, dry with anhydrous sodium sulfate, and concentrate under reduced pressure to obtain a white solid. The obtained crude product was slurried with 200 ml of petroleum ether and filtered under reduced pressure to obtain 35.7 g of white solid, with an LC purity of more than 55% and a yield of 80%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com