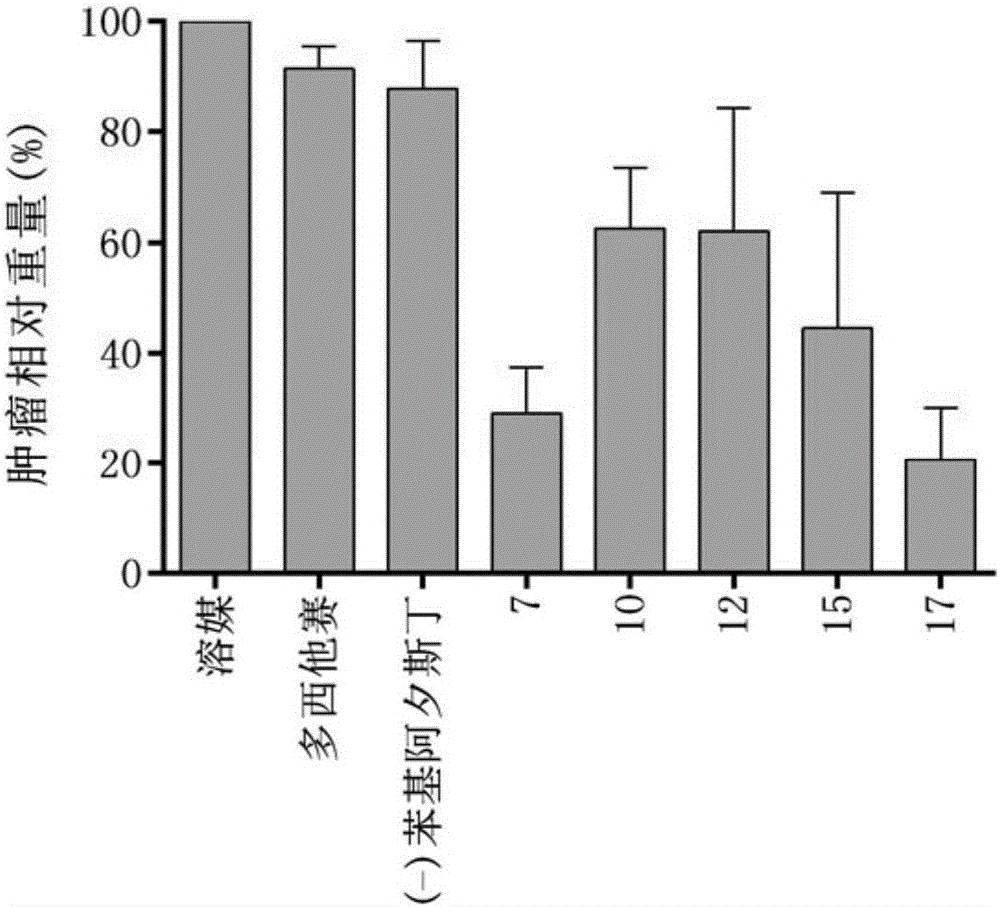
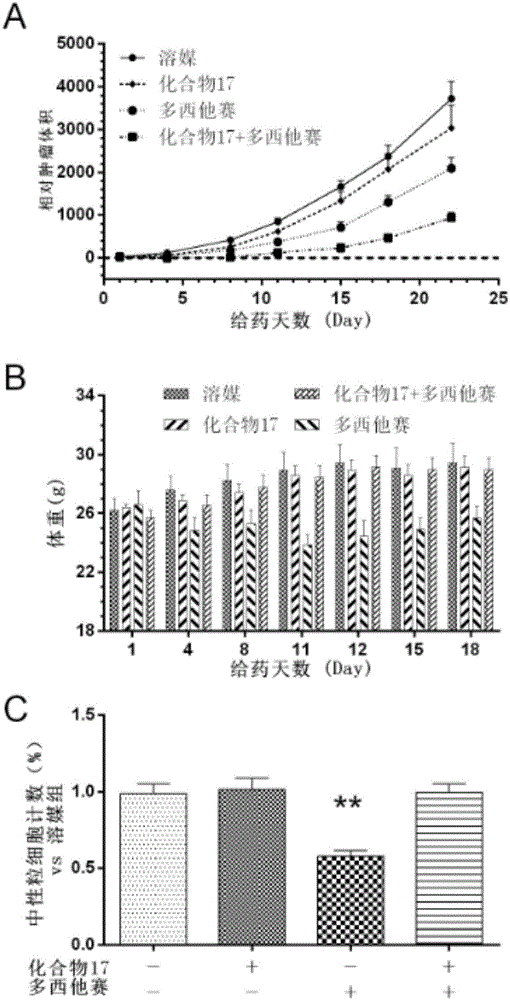
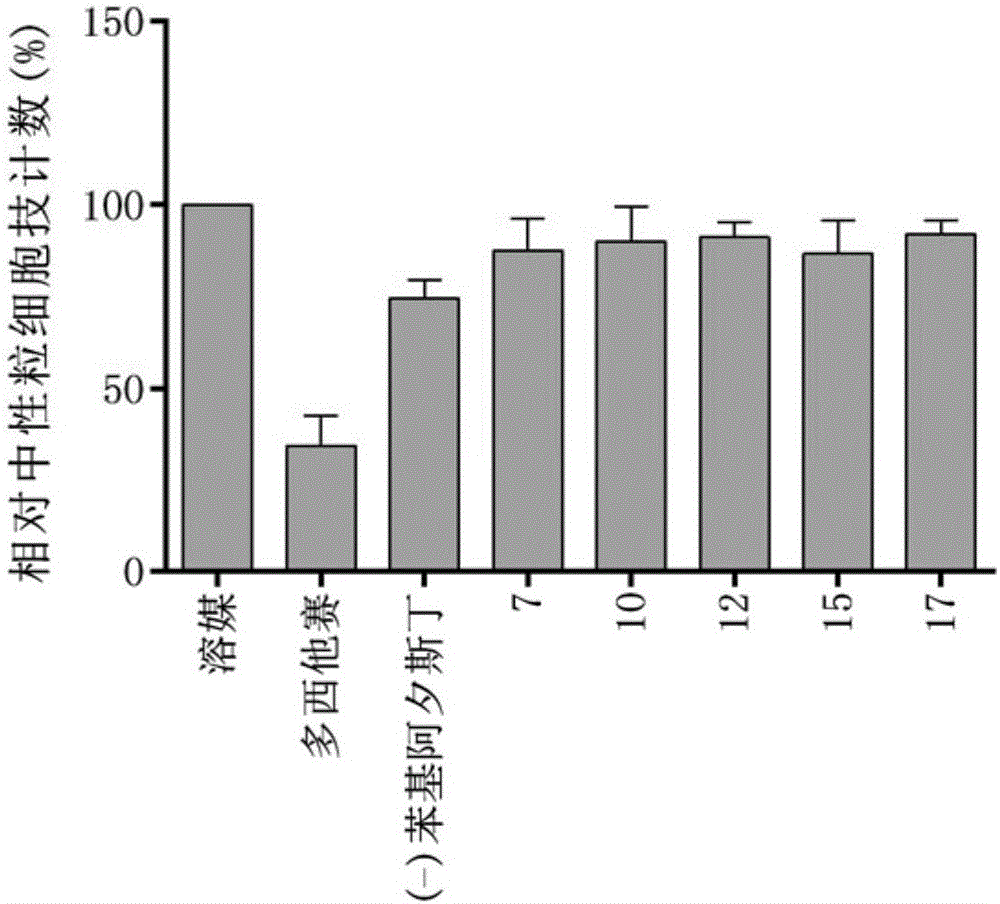
Tubulin inhibitor
A C1-C20, compound technology, applied in the directions of medical preparations containing active ingredients, organic active ingredients, organic chemistry, etc., can solve the problems of large interference of immune system function and weak tumor inhibitory effect, and achieve increased tumor inhibitory effect. , Little interference with immune system function, inhibition of growth and proliferation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] step 1:
[0059]
[0060] Compound A was synthesized by referring to the method described in Route A of Example 2 in Chinese Patent CN1684955.
[0061] Step 2:
[0062]
[0063] 4-Trifluoromethoxybenzaldehyde 3 (19.0 g, 0.10 mol), formylmethylenetriphenylphosphine 4 (33.5 g, 0.11 mmol) and toluene (200 ml) were added to a 1 L dry one-necked flask. The reaction was refluxed for 17 hours and then concentrated. The crude product was purified by column chromatography (eluent: volume ratio of petroleum ether / ethyl acetate: 100 / 1 to 80 / 20) to obtain yellow oil 5 (14.9g, yield: 69%).ESI-MS:m / z=217.1 (M+H).
[0064] Step 3:
[0065]
[0066] DMF (100ml), Intermediate A (1.38g, 5mmol), 3-(4-trifluoromethoxyphenyl)acrolein 5 (2.16g, 10mmol) and cesium carbonate (3.26g) were added to a 250ml dry single-necked flask ,10mmol). The reaction system was stirred at 25°C for 12 hours, cooled to room temperature, and then poured into ice water. Extract with ethyl acetate,...
Embodiment 2~11
[0068] Steps 1-2 prepare intermediate A, the same as Steps 1-2 in Example 1. The preparation of other intermediates and final products is shown in the table below:
[0069] Table 2: Preparation methods of other compounds
[0070]
[0071]
[0072]
[0073]
[0074]
Embodiment 14
[0075] Embodiment 14: Cell level inhibition experiment
[0076] Purpose of the experiment: Detect 14 candidate compounds against 5 cell lines using CCK-8 detection kit
[0077] (MDA-MB-231, HT-1080, AGS, A-673 & HUVEC) cytotoxic IC50 value.
[0078] Materials and methods:
[0079] Cell line:
[0080] MDA-MB-231 human breast cancer cell line (ordered from Shanghai Cell Resource Center, Chinese Academy of Sciences)
[0081] HT-1080 human fibrosarcoma cell line (ordered from Shanghai Cell Resource Center, Chinese Academy of Sciences)
[0082] AGS human gastric adenocarcinoma cell line (ordered from Shanghai Cell Resource Center, Chinese Academy of Sciences)
[0083] A-673 human rhabdomyosarcoma cell line (ordered from Shanghai Cell Resource Center, Chinese Academy of Sciences)
[0084] HUVEC human umbilical vein endothelial cell line (ordered from ScienCell Research Laboratories, Cat#8000, Lot#11233)
[0085] Reagents and Consumables:
[0086] Cell Counting Kit-8 (Cat#CK04...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com