Anticancer pharmaceutical composition, preparation thereof and preparing method
An anti-cancer drug and composition technology, applied in the field of anti-tumor drug research, to achieve the effects of reducing toxic side effects, improving bioavailability and druggability, and reducing IC50
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Example 1 Preparation of Anticancer Drug Composition A
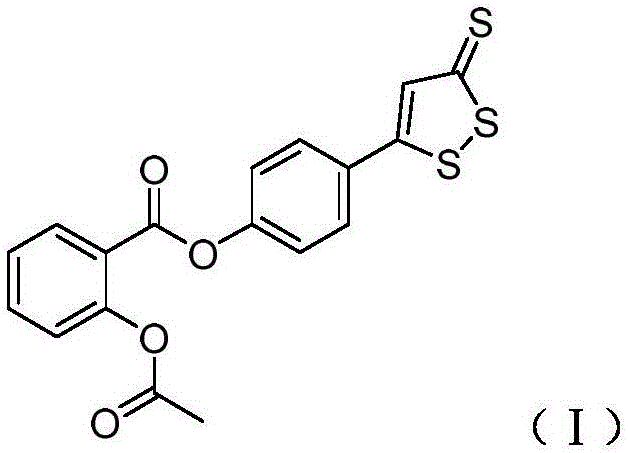
[0036] Synthesis of aspirin derivatives that release hydrogen sulfide:
[0037]
[0038] As shown in the above reaction formula (a), 5.6 grams of compound 1 and 5.8 grams of compound 2 were added to 240 milliliters of dichloromethane, and then 0.25 grams of DMAP was added, stirred and reacted at room temperature for 6 hours, and the solvent was removed by vacuum rotary evaporation , followed by purification by column chromatography to obtain 10.9 g of compound 3.
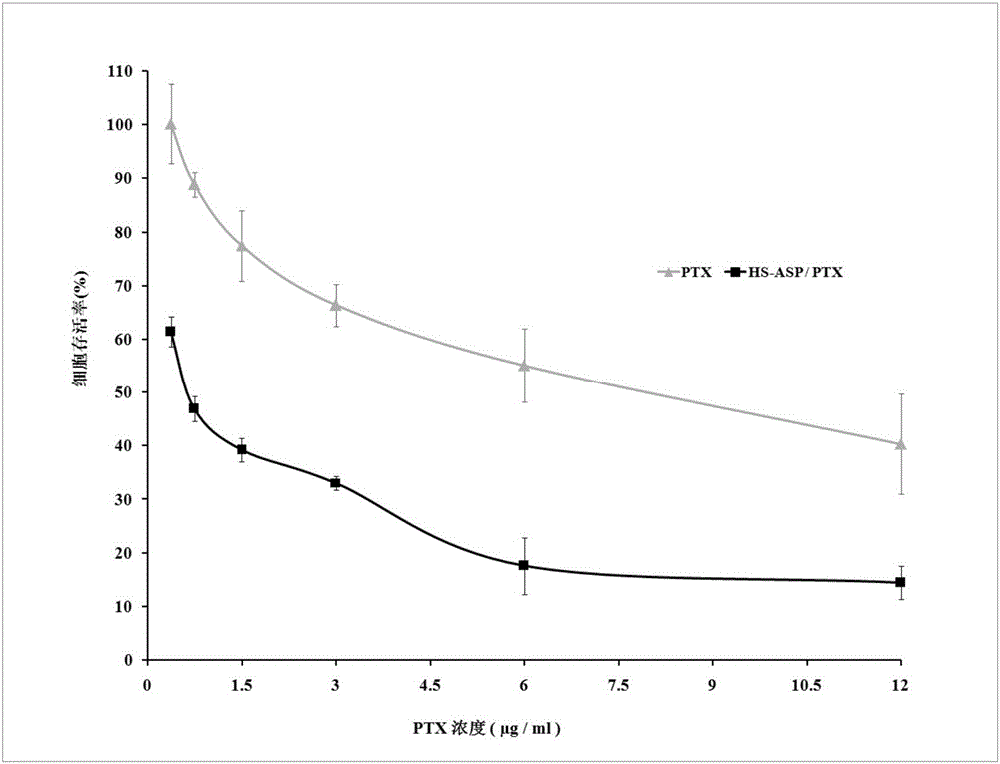
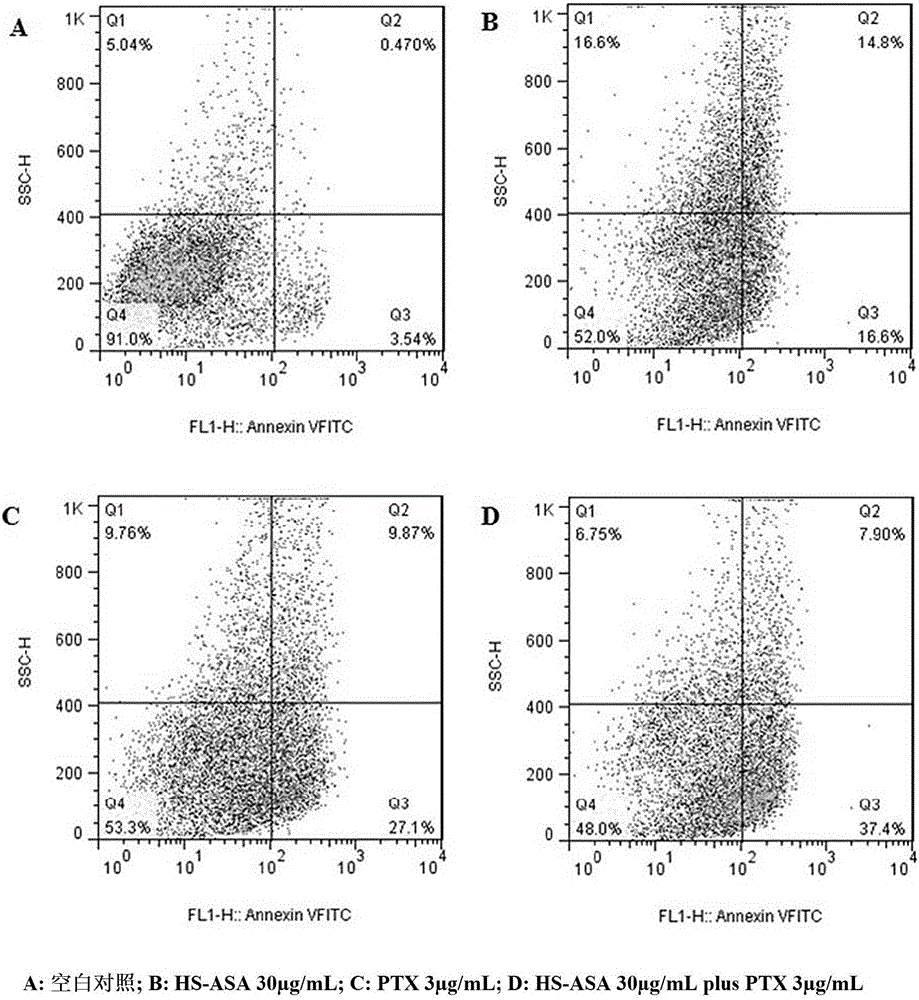
[0039] The anticancer drug composition A is obtained by mixing paclitaxel with the compound 3 synthesized above in a weight ratio of 1:5.
Embodiment 2
[0040] Example 2 Preparation of Anticancer Drug Composition B
[0041] Synthesis of aspirin derivatives that release hydrogen sulfide:
[0042]
[0043] As shown in the above reaction formula (a), 10.2 grams of compound 1 and 9.8 grams of compound 2 were added to 420 milliliters of dichloromethane, and then 0.48 grams of DMAP was added, stirred and reacted at room temperature for 6 hours, and the solvent was removed by vacuum rotary evaporation , followed by purification by column chromatography to obtain 18.6 g of compound 3.
[0044] The anticancer drug composition B is obtained by mixing paclitaxel with the compound 3 synthesized above in a weight ratio of 1:1.
Embodiment 3
[0045] Example 3 Preparation of Anticancer Drug Composition C
[0046] Synthesis of aspirin derivatives that release hydrogen sulfide:
[0047]
[0048] As shown in the above reaction formula (a), 7.6 grams of compound 1 and 7.2 grams of compound 2 were added to 360 milliliters of dichloromethane, and then 0.36 grams of DMAP was added, stirred and reacted at room temperature for 6 hours, and the solvent was removed by vacuum rotary evaporation , followed by purification by column chromatography to obtain 15.4 g of compound 3.
[0049] The anticancer drug composition C is obtained by mixing paclitaxel with the compound 3 synthesized above at a weight ratio of 1:10.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com