Use of oxycaine in preparation of medicine and pharmaceutical composition
A technology for the preparation of oxycaine and drugs, which is applied in the field of biomedicine, can solve the problems that oxycaine needs to be further developed, and achieve the effects of low toxicity, high bioavailability, and clear metabolic pathways
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
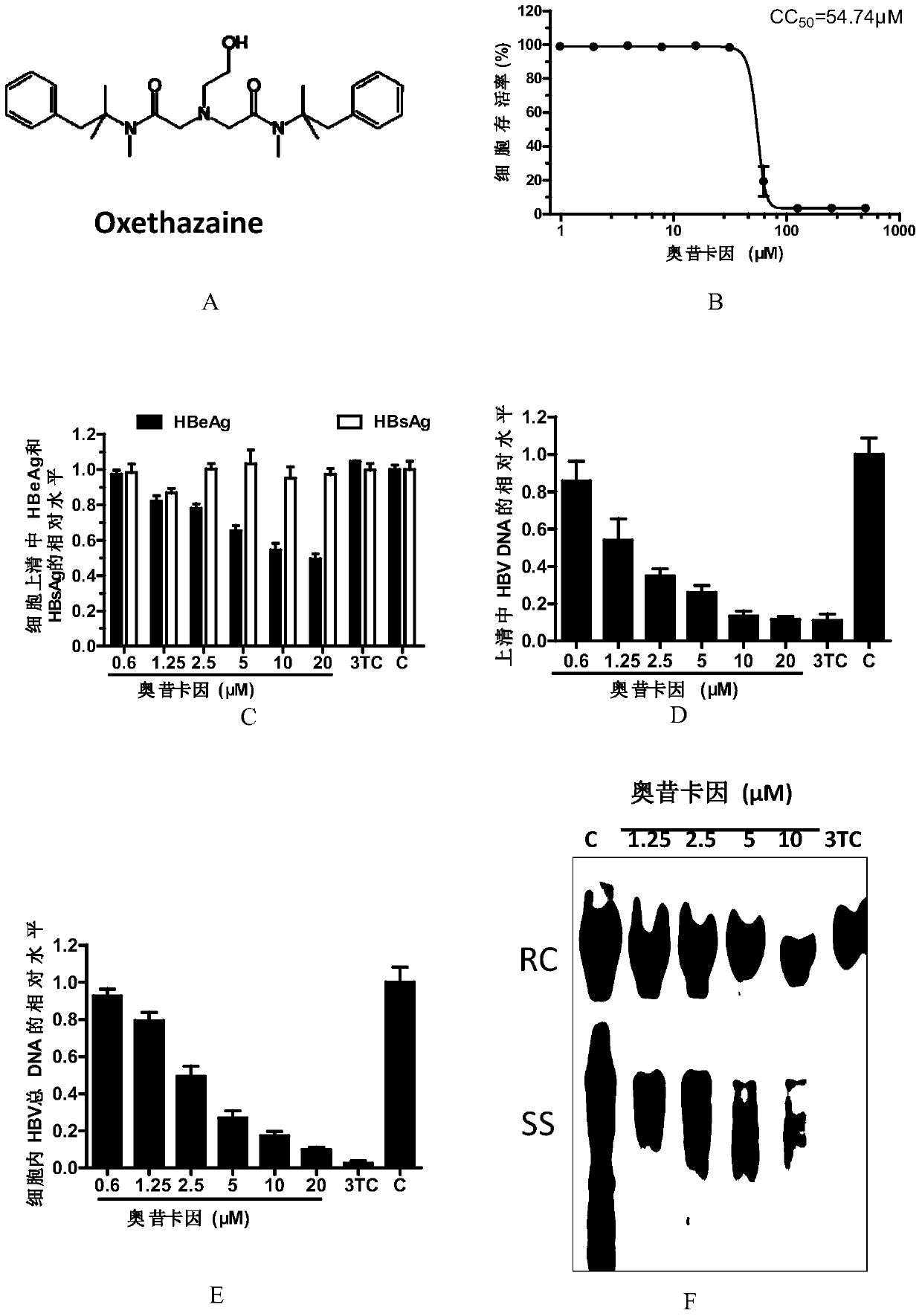
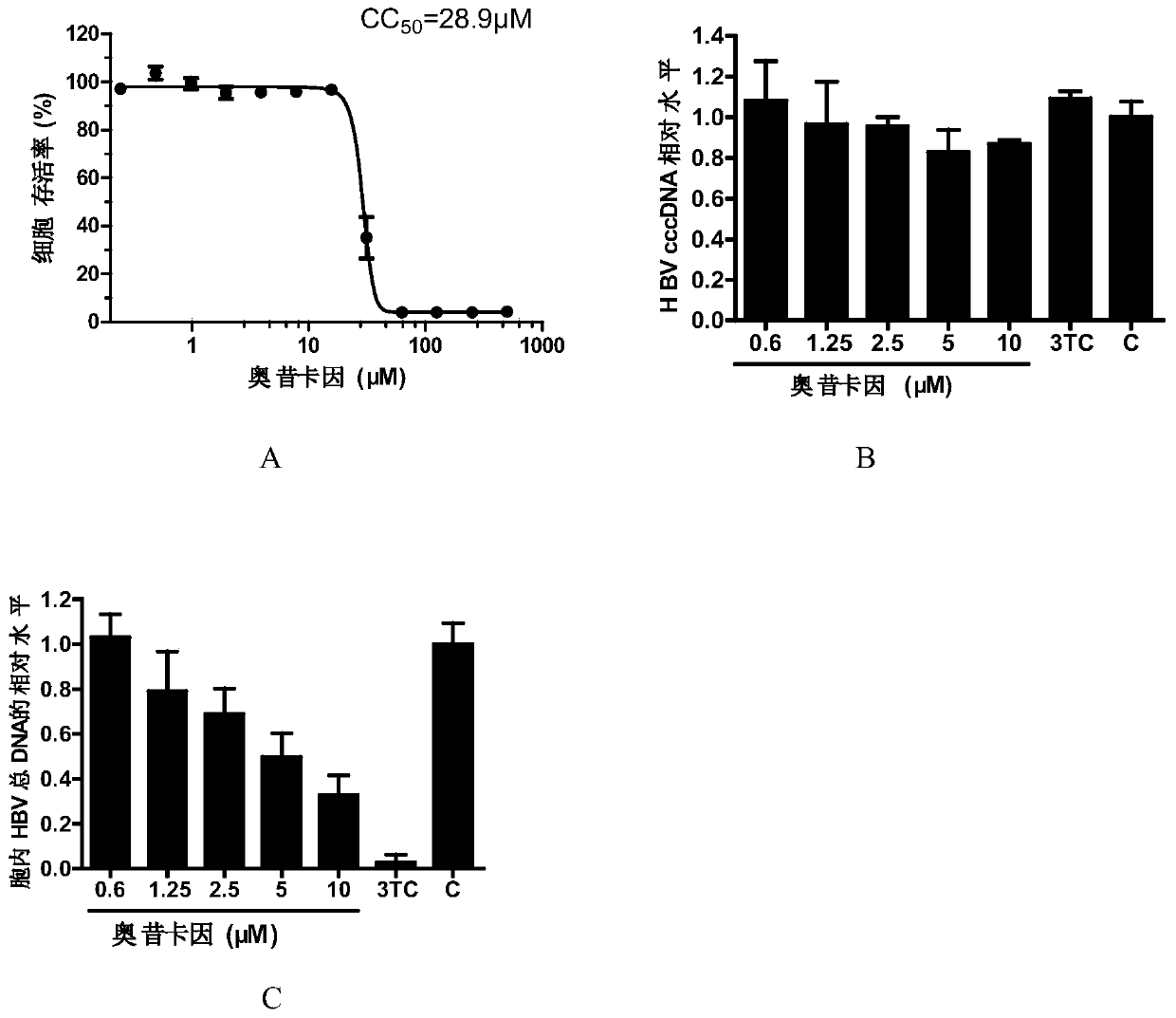
[0047] The evaluation of embodiment 1 oxycaine anti-HBV activity
[0048] 1. Experimental materials
[0049] 1.1 Cells and Drugs
[0050] HepAD38 cell line: Human liver cancer stable transfection cell line, the HBV genome is integrated in its chromosome, which can support HBV DNA synthesis and protein expression, and can produce infectious virus particles. Viral replication in this cell line is controlled by the tetracycline-regulated CMV promoter.
[0051] Oxycaine was purchased from Sigma, and lamivudine (3TC) was a gift from NIH.
[0052] 1.2 Reagents
[0053] DMEM / F12 medium and fetal bovine serum (FBS) were purchased from GIBCO; HBV surface antigen (S antigen) and e antigen detection kits were purchased from Shanghai Kehua Biotechnology Co., Ltd.; Reagents were purchased from Invitrogen; iTaqUniversal SYBR Green supermix reagent was purchased from Bio-rad; DIG High Prime DNA Labeling and Detection Starter Kit II was purchased from Roche.
[0054] 2. Experimental met...
Embodiment 2
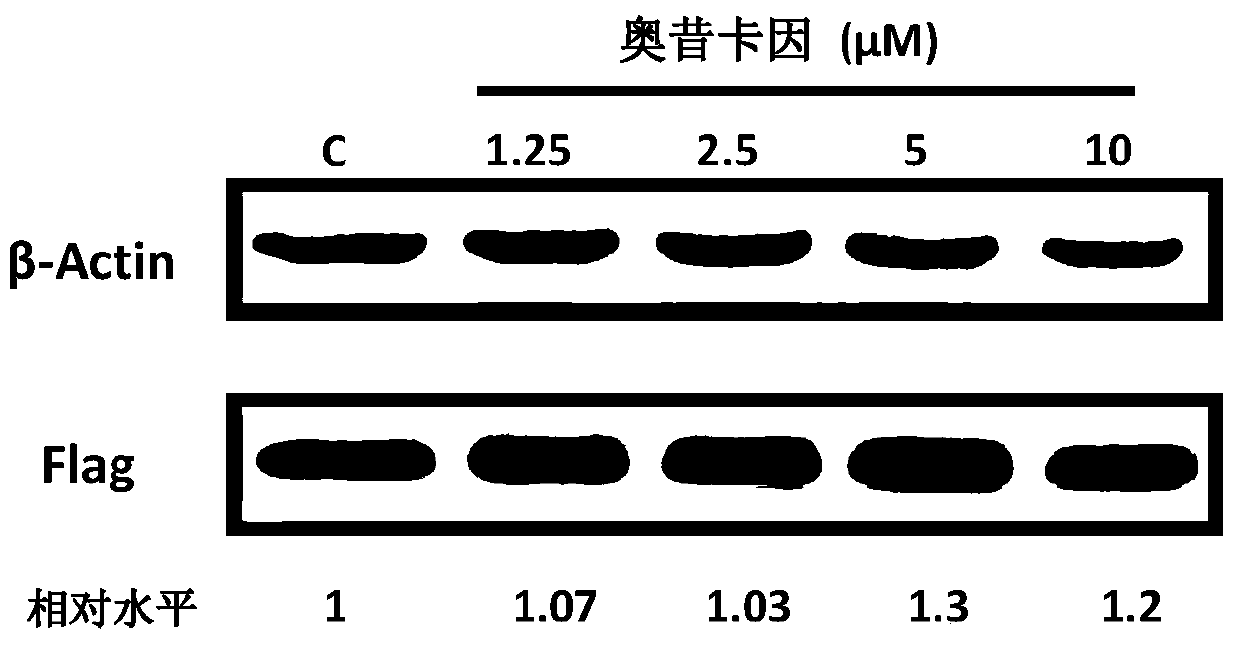
[0076] Example 2 Oxethazaine directly acts on HBV nucleocapsid assembly to exert antiviral effect
[0077] 1. Experimental materials
[0078] 1.1 Cells, plasmids and drugs
[0079] HepAD38 cell line: Human liver cancer stable transfection cell line, the HBV genome is integrated in its chromosome, which can support HBV DNA synthesis, protein expression, and produce infectious virus particles. Viral replication in this cell line is controlled by the tetracycline-regulated CMV promoter.
[0080] Huh7 is a human liver cancer cell, donated by Chen Xinwen, a researcher at Wuhan Institute of Virology.
[0081] pFlag-GAPDH is a plasmid expressing the GAPDH protein fused with the Flag tag under the control of the CMV promoter constructed in our laboratory;
[0082] Oxycaine was purchased from Sigma, and lamivudine (3TC) was a gift from NIH.
[0083] 1.2 Reagents
[0084] MEM and DMEM / F12 medium and fetal bovine serum (FBS) were purchased from GIBCO; Reagents and transfection rea...
Embodiment 3
[0126] Embodiment 3 Oxethazaine (Oxethazaine) is to the antiviral effect of nucleoside analog drug-resistant HBV mutant strain
[0127] 1. Experimental materials
[0128] 1.1 Cells, plasmids and drugs
[0129] Huh7 is a human liver cancer cell;
[0130] Plasmid REZ31-9-1 (59#) contains 1.1 times the wild-type HBV genome sequence; adefovir (ADV) resistant mutant strain expression plasmid REZ36-10-1 (61#), which contains 1.1 times the N236T mutation HBV genome sequence; lamivudine (3TC) and entecavir (ETV) double-resistant mutant strain expression plasmid REZ7-8-4 (70#), which contains 1.1 times the HBV genome sequence of L180M+M204V+S202G triple mutation. The above plasmids were donated by Chen Xinwen, researcher of Wuhan Institute of Virology.
[0131] Oxycaine was purchased from Sigma, lamivudine (3TC) was a gift from NIH; adefovir (ADV) and entecavir (ETV) were purchased from Dalian Meilun Biotechnology Co., Ltd.
[0132] 1.2 Reagents
[0133] DMEM medium and fetal bovi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com