Method for rapidly disposing isopropyl nitrate and application of method
A technology of isopropyl nitrate and a processing method, which is applied in the field of safe processing of nitrate-based energetic materials, can solve the problems of undisclosed safe processing methods of isopropyl nitrate energetic materials, etc., and achieves reaction rate improvement and simple reaction conditions. , the effect of high removal rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-16
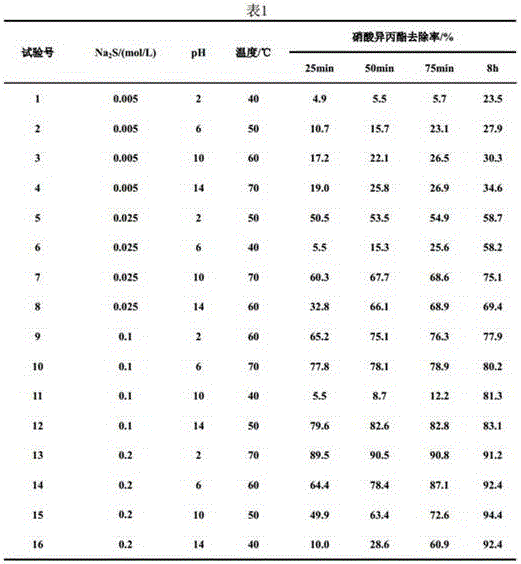
[0021] Prepare 150mL sodium sulfide solutions of different concentrations, add them to a constant temperature water bath, raise the temperature to the corresponding temperature, then adjust the pH value, add 5mL isopropyl nitrate, and start the reaction. Take 10 mL of the reaction product at 25 min, 50 min, 75 min, and 8 h of reaction, extract with 10 mL of diethyl ether, analyze the extraction phase by gas chromatography, and calculate the removal rate of isopropyl nitrate.
[0022] The concentration of sodium sulfide solution, reaction temperature, reaction pH value and removal rate of isopropyl nitrate in each embodiment are shown in Table 1 below.
[0023]
[0024] As can be seen from Table 1, the preferred reaction conditions of isopropyl nitrate and excessive sodium sulfide reaction are:
[0025] A sodium sulfide aqueous solution with a concentration of 0.1-0.2 mol / L is used, the pH of the reaction solution is controlled to be 6-14, and the temperature is 50-70°C. In...
Embodiment 17-20
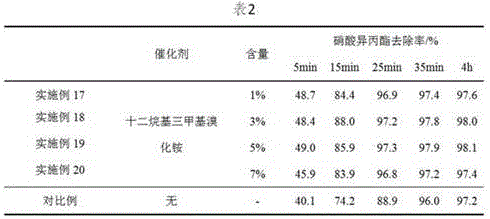
[0029] Prepare 150mL of sodium sulfide solution with a concentration of 0.2mol / L, add it to the reaction kettle, raise the temperature to 70°C, adjust the pH value to 10, add 5mL of isopropyl nitrate to be decomposed, and add a corresponding amount of catalyst to start the reaction.
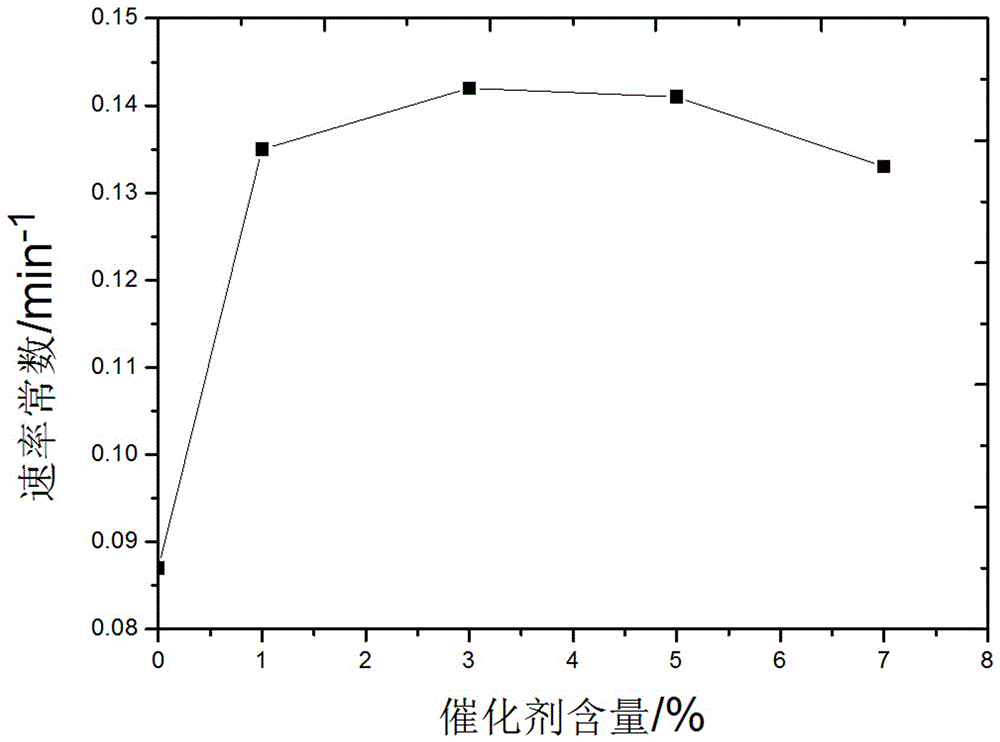
[0030] The amount that adds catalyst among the embodiment 17-20 is respectively 1%, 3%, 5%, 7% of isopropyl nitrate molar weight, does not add dodecyltrimethylammonium bromide in the comparative example, with this Consider the effect of the amount of catalyst on the reaction. At 5min, 15min, 25min, 35min, and 4h of the reaction, 10mL of the reaction product was taken, extracted with 10mL of ether, and the extraction phase was analyzed by gas chromatography to determine the removal rate of isopropyl nitrate. By comparison, the influence of the catalyst on the reaction was determined. Table 2 is the test results. figure 1 A plot of the rate constants measured for different experimental reactions....
Embodiment 21-24
[0037] With reference to embodiment 17-20, in embodiment 21-24, control the reaction temperature of sodium sulfide aqueous solution and isopropyl nitrate to be 60 ℃, the amount of adding catalyst is 5% of isopropyl nitrate molar weight, all the other reaction control conditions and The removal rate of isopropyl nitrate measured in different time periods is shown in Table 3 below.
[0038]
[0039] By table 3 and table 1 isopropyl nitrate removal ratio comparison shows that catalyst dodecyltrimethylammonium bromide catalyzes sodium sulfide aqueous solution and isopropyl nitrate reaction, can significantly improve reaction rate constant, and in a short period of time is A high removal rate of isopropyl nitrate can be obtained.
[0040] The invention also discloses the application of the above method, which is applied to the scrap treatment of weapons and ammunition containing isopropyl nitrate. For example, for the cloud explosion bomb, isopropyl nitrate is the main componen...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com