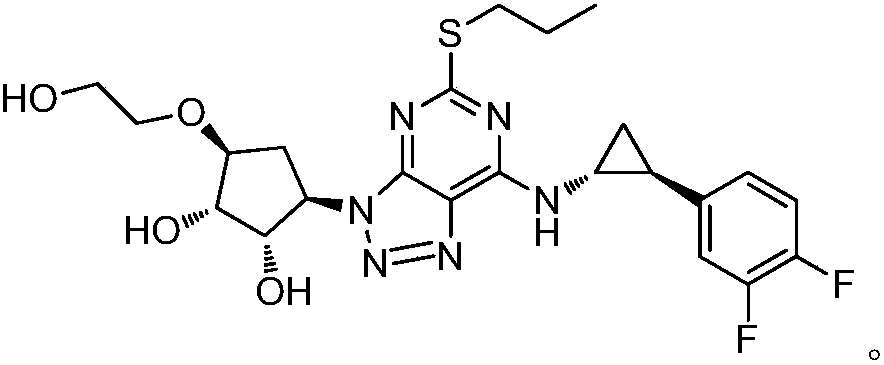
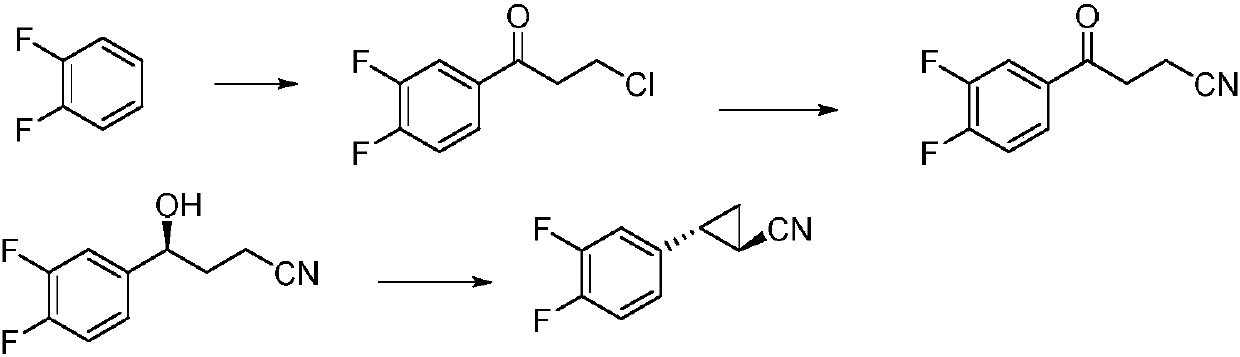
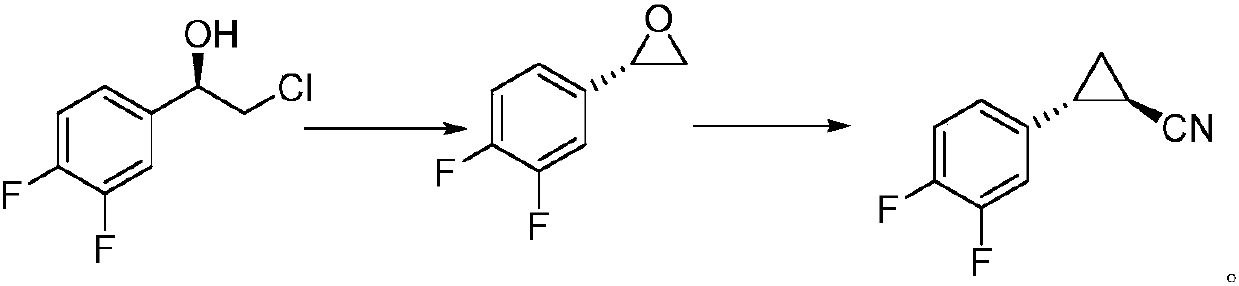
A kind of preparation technology of (1r, 2s)-1-cyano-2-(3,4-difluorophenyl) cyclopropane
A technology for the preparation of difluorophenyl, which is applied in the field of preparation of ticagrelor intermediate 1-cyano-2-cyclopropane, achieving high stereoselectivity, high overall yield, and easy operation of the preparation process Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Preparation of (2S)-2-(3,4-difluorophenyl)oxirane
[0027] Under the protection of nitrogen, mix (S)-binaphthol phosphate 7g (20mmol) and 3,4-difluorostyrene 14.0g (100mmol) in tetrahydrofuran for 10-15min, then keep 20°C and add hydrogen peroxide (30% , 300mmol), continue to maintain the temperature and stir the reaction for 4 hours. After the reaction is completed, add water to the reaction solution, separate layers, wash the organic layer with water, concentrate under reduced pressure, and recrystallize petroleum ether to obtain (2S)-2-(3,4-difluoro Phenyl)oxirane 14.7g, yield 94.1%, ee value 99.15%. 1 H NMR (400MHz, CDCl3) δ: 7.03-7.28 (m, 4H), 3.81-3.84 (m, 1H), 3.10-3.15 (m, 1H), 2.69-2.72 (dd, J=2.45Hz, 1H).
Embodiment 2
[0029] Preparation of (2S)-2-(3,4-difluorophenyl)oxirane
[0030] Under the protection of nitrogen, mix 20.9g (60mmol) of (S)-binaphthol phosphate and 28g (200mmol) of 3,4-difluorostyrene in tetrahydrofuran for 10-15min, then add hydrogen peroxide (30% , 500mmol), continue to maintain the temperature and stir the reaction for 3 hours. After the reaction is completed, add water to the reaction solution, separate layers, wash the organic layer with water, concentrate under reduced pressure, and recrystallize petroleum ether to obtain (2S)-2-(3,4-difluoro Phenyl)oxirane 14.6g, yield 93.2%, ee value 99.01%.
Embodiment 3
[0032] Preparation of (2S)-2-(3,4-difluorophenyl)oxirane
[0033] Under nitrogen protection, mix 7g (20mmol) of (S)-binaphthol phosphate and 14g (100mmol) of 3,4-difluorostyrene in tetrahydrofuran for 10-15min, then add 142.0g (30mmol) of hydrogen peroxide dropwise at 10°C %, 200mmol), continue to maintain the temperature and stir the reaction for 4 hours, after the reaction is completed, add water to the reaction solution, separate layers, wash the organic layer with water, concentrate under reduced pressure, and recrystallize petroleum ether to obtain (2S)-2-(3,4-di Fluorophenyl)oxirane 14.9g, yield 95.5%, ee value 98.92%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com