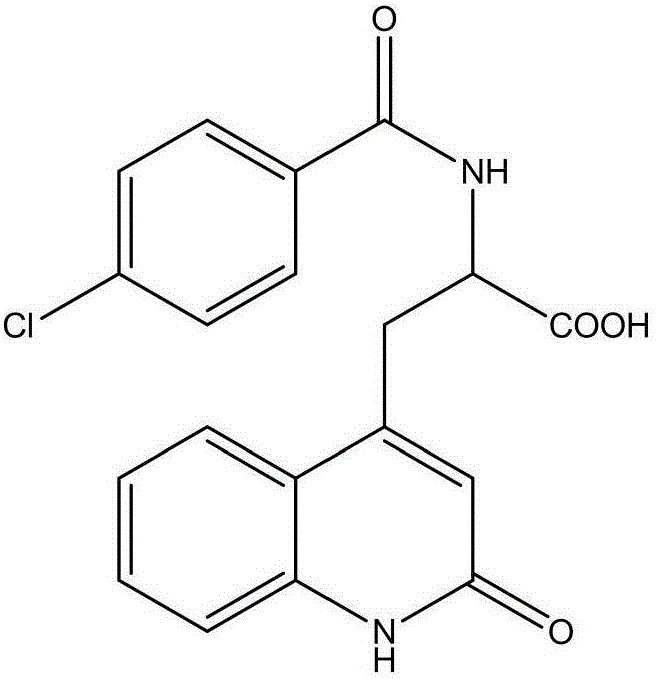
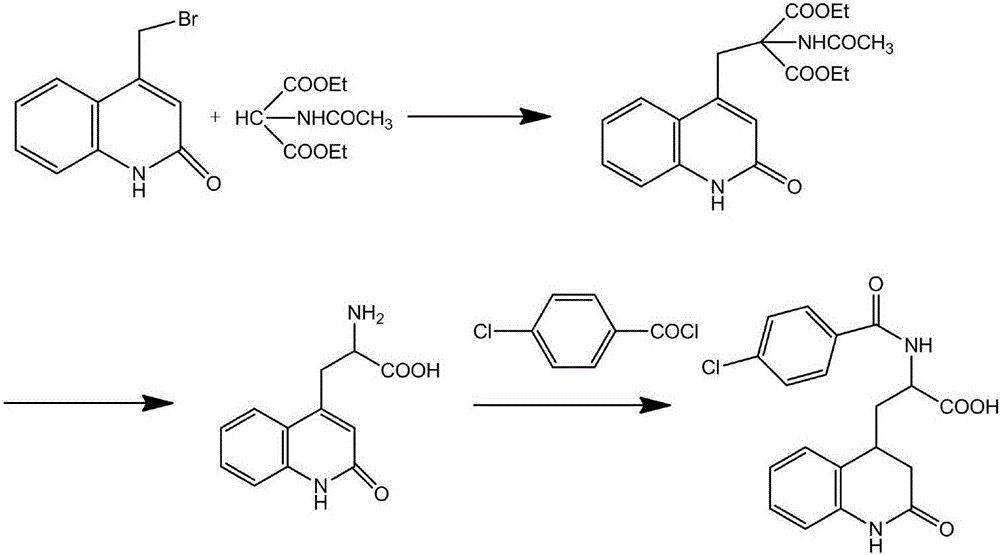
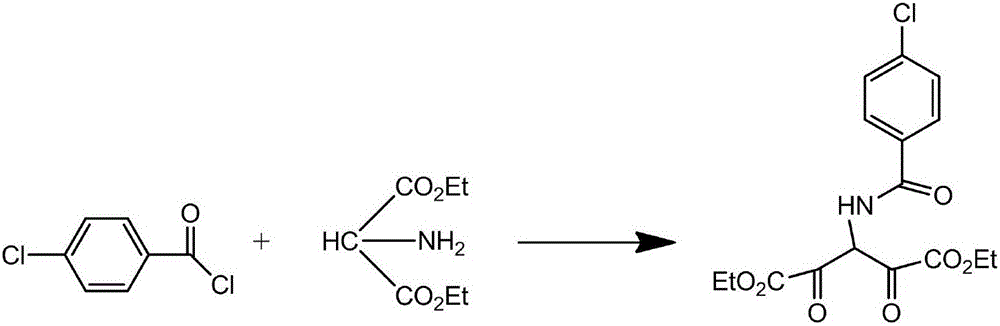
Process for synthesizing rebamipide
A synthesis process and technology of rebamipide, applied in the field of synthesis technology of rebamipide, can solve the problems of high impurity content, low product purity, difficulty in obtaining high-purity rebamipide, etc., and achieve the effect of high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0020] In order to make the purpose, technical solutions and advantages of the embodiments of the present invention clearer, the technical solutions in the embodiments of the present invention will be clearly and completely described below in conjunction with the embodiments of the present invention. Based on the embodiments of the present invention, all other embodiments obtained by persons of ordinary skill in the art without creative efforts fall within the protection scope of the present invention.
[0021] The synthetic technique of rebamipide, the steps are as follows:
[0022] 1) Add 10g diethyl aminomalonate and 10g triethylamine to 200ml chloroform, stir at room temperature for 30min, then cool to 5°C, add 8.2g 4-chlorobenzoyl chloride dropwise to the reaction solution , after the dropwise addition, react at room temperature for 2 hours, spot the plate to determine the end of the reaction, wash the reaction solution with 200ml of water, 40ml of 5% sodium carbonate sol...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| quality score | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com