Fluorescent probes for detecting formaldehyde, and preparation and application thereof
A fluorescent probe and formaldehyde technology, applied in the field of environmental analytical chemistry and biological detection, can solve the problems of slow detection response, disturbance of endogenous formaldehyde concentration, poor sensitivity, etc., and achieve fast detection response, good stability, and good sensitivity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Embodiment 1: Preparation of fluorescent probe molecule I-1
[0027] 7-Diethylaminocoumarin (500 mg) and Lawson's reagent (1.86 g) were refluxed in dry toluene for 6 hours. After the reaction, cool and filter, spin the filtrate to dry the solvent, dissolve it in ethanol, add 80% hydrazine hydrate, and react under reflux for 3 hours. After the reaction was completed, the solvent was spin-dried, water was added, extracted three times with dichloroformaldehyde, the organic phases were combined, dried over anhydrous sodium sulfate, and purified by column chromatography to obtain I-1 (65% yield). LC-MS: m / z 232.3010[M+H] + .
[0028]
Embodiment 2
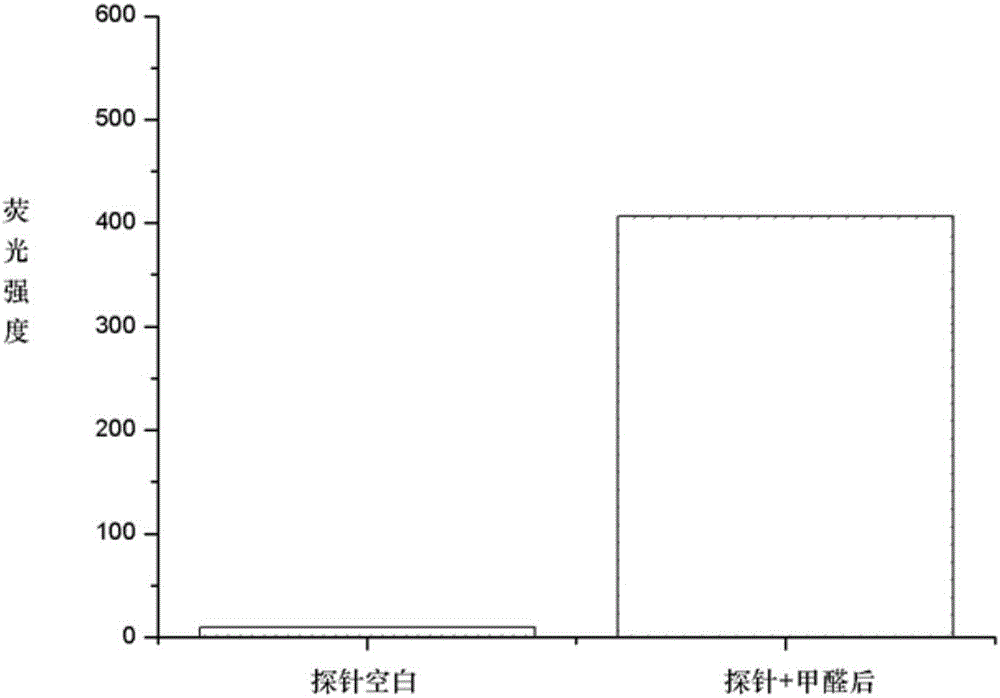
[0029] Embodiment 2: the preparation of fluorescent probe molecule II-1
[0030] 7-Methoxycoumarin (500 mg) and Lawson's reagent (1.86 g) were refluxed in dry toluene for 6 hours. After the reaction, cool and filter, spin the filtrate to dry the solvent, dissolve it in ethanol, add 80% hydrazine hydrate, and react under reflux for 3 hours. After the reaction was completed, the solvent was spin-dried, water was added, extracted three times with dichloroformaldehyde, the organic phases were combined, dried over anhydrous sodium sulfate, and purified by column chromatography to obtain II-1 (yield 70%). LC-MS: m / z 191.2066[M+H] + .
Embodiment 3
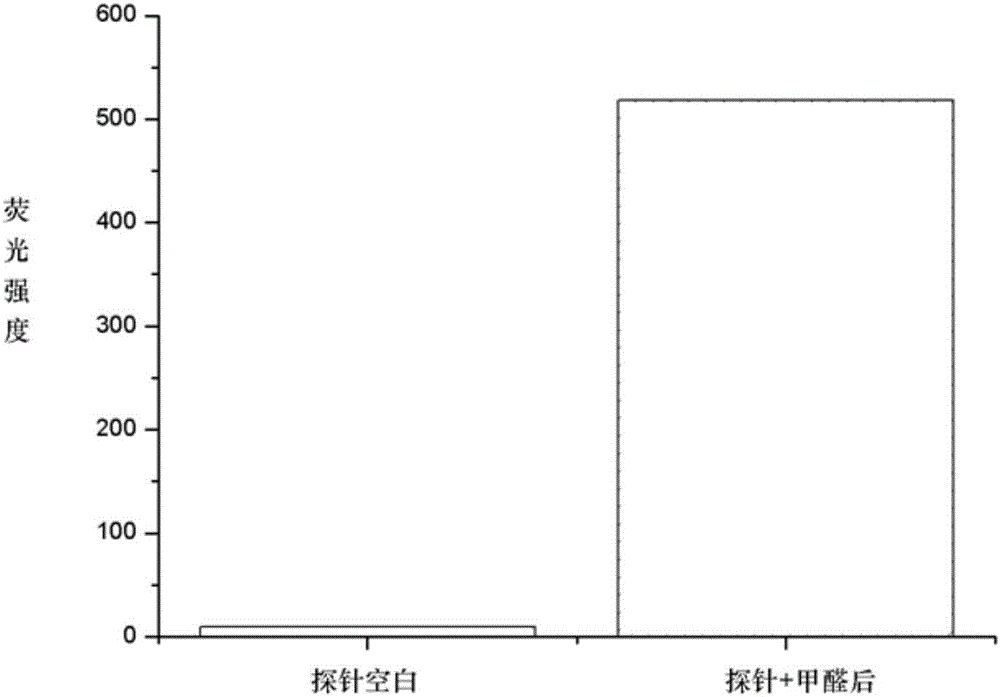
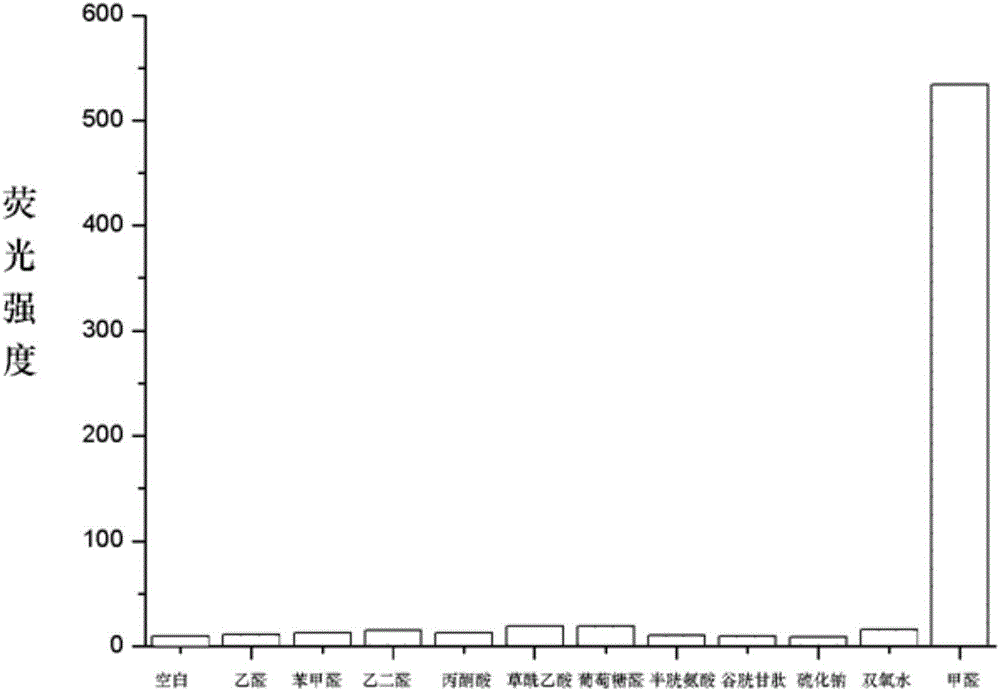
[0031] Embodiment 3: Fluorescence changes before and after the reaction of fluorescent probe molecule I-1 with formaldehyde
[0032] Dissolve the probe molecule in a small amount of DMSO, and add PBS buffer or formaldehyde in PBS solution respectively, so that the final concentration of the probe molecule is 10 μM, and the final concentration of formaldehyde is 500 μM. After reacting for 10 minutes, use a fluorescence spectrometer to excite at 450nm, record the fluorescence intensity of the solution at the maximum emission wavelength (about 500nm), and then confirm that the fluorescence intensity of the probe molecule is enhanced after the reaction with formaldehyde, such as figure 1 shown.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com