Isomannitol-bisbenzimidazole salt compound and preparation method thereof
A technology for isomannitol and bisbenzimidazole, which is applied in the field of isomannitol-bisbenzimidazole salt compounds and their preparation, and can solve problems such as higher requirements for medicines
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
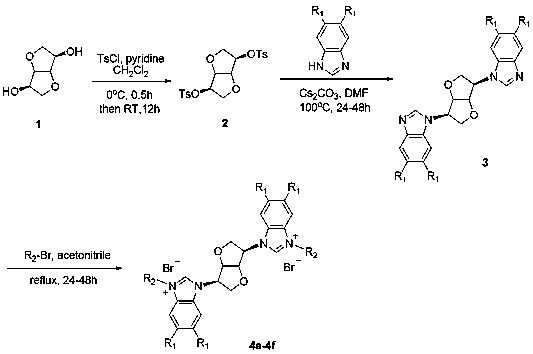
[0016] The preparation method specifically includes:
[0017] A, the preparation of compound isomannitol p-toluenesulfonate:
[0018] Using isomannitol as raw material, synthesize isomannitol p-toluenesulfonate with p-toluenesulfonyl chloride in dichloromethane: add isomannitol to dichloromethane to dissolve it completely, then add anhydrous pyridine, 0 Add p-toluenesulfonyl chloride dropwise under the condition of ℃, the dosage is isomannitol: anhydrous pyridine: p-toluenesulfonyl chloride = 1: 4.0: 2.5, the dosage of dichloromethane is 25-50 ml: 1g isomannitol Mannitol, after all the addition is complete and completely dissolved, rise to room temperature, stir the reaction for 12 h until the thin layer chromatography shows that the raw material is completely reacted, add dichloromethane to dilute (50 ml: g substrate), and then use 1N hydrochloric acid ( 50 ml) and saturated brine (50 ml), the organic phase was washed with anhydrous NaSO 4 After drying, filtering, and conce...
Embodiment 1
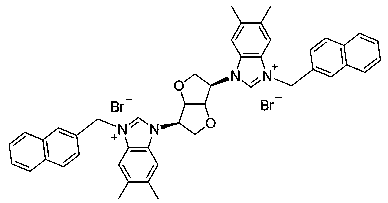
[0024] Preparation of Compound 4a: See Preparation Methods A, B, and C above.
[0025]
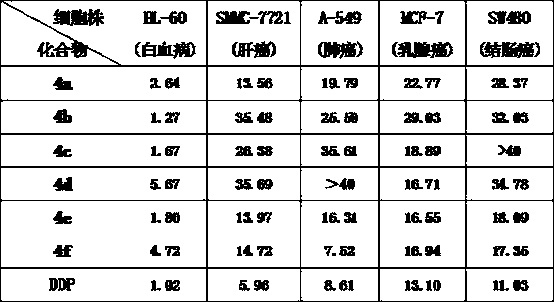
[0026] Compound 4a: Formula C 34 h 30 Br 4 N 4 o 2 ,yield 67%. White solid powder, mp 151-153 o c. 1 HNMR (300 MHz, DMSO-d 6 ): δ 10.12 (2H, s), 8.27 (1H, s), 8.24 (1H, s), 7.83-7.66 (10H, m), 7.39-7.32 (6H, m), 5.91-5.89 (4H, m) , 5.80 (2H, t, J = 3.9Hz), 5.45 (2H, s), 4.64 (4H, d, J = 3.6 Hz). 13 C NMR (75 MHz, DMSO-d 6 ): δ143.83, 142.26, 133.31, 133.15, 132.52, 131.13, 131.05, 130.91, 130.85,130.73, 129.95, 128.46, 128.31, 127.26, 127.12, 123.03, 122.56, 114.12,114.04, 113.88, 86.43, 70.82, 62.53, 50.53. HRMS (ESI-TOF) m / z Calcd for C 34 h 29 Br 2 N 4 o 2 + [M-2Br-H] + 683.0651, found 683.0647.
Embodiment 2
[0028] Preparation of Compound 4b: See Preparation Methods A, B, and C above.
[0029]
[0030] Compound 4b: Formula C 36 h 36 Br 2 N 4 o 2 , Yield 83%. White solid powder, mp 263-264 o c. 1 HNMR (300 MHz, DMSO-d 6 ): δ 10.28-10.22 (2H, m), 8.22 (1H, s), 8.20 (1H, s),7.93-7.91 (2H, m), 7.75-7.62 (4H, m), 7.48-7.47 (4H, m), 7.21-7.19 (4H, m), 5.78 (6H, s), 5.48 (1H, s), 5.46 (1H, s), 4.65 (4H, s), 2.28 (6H, d, J = 4.8Hz ). 13 C NMR (75 MHz, DMSO-d 6): δ 142.40, 141.25, 138.17, 138.06, 131.02,130.90, 130.77, 130.60, 129.50, 129.35, 128.31, 126.96, 126.70, 126.12,114.41, 114.13, 113.97, 86.37, 70.80, 62.48, 49.89, 49.80, 20.68. HRMS (ESI-TOF) m / z Calcd for C 36 h 35 N 4 o 2 + [M-2Br-H] + 555.2754, found 555.2757.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com