Method for producing oxidized gamma-glutamylcysteine and oxidized glutathione
A technology of glutamylcysteine and glutathione, applied in biochemical equipment and methods, ligases, enzymes, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0079] The base sequence of the gene (DNA or RNA) encoding GSH I that can be used in the preparation of GSH I is not limited to the base sequence shown in SEQ ID NO: 1, and may be the amino acid sequence encoding the target GSH I, An appropriate base sequence corresponding to the type of host organism.
[0080]
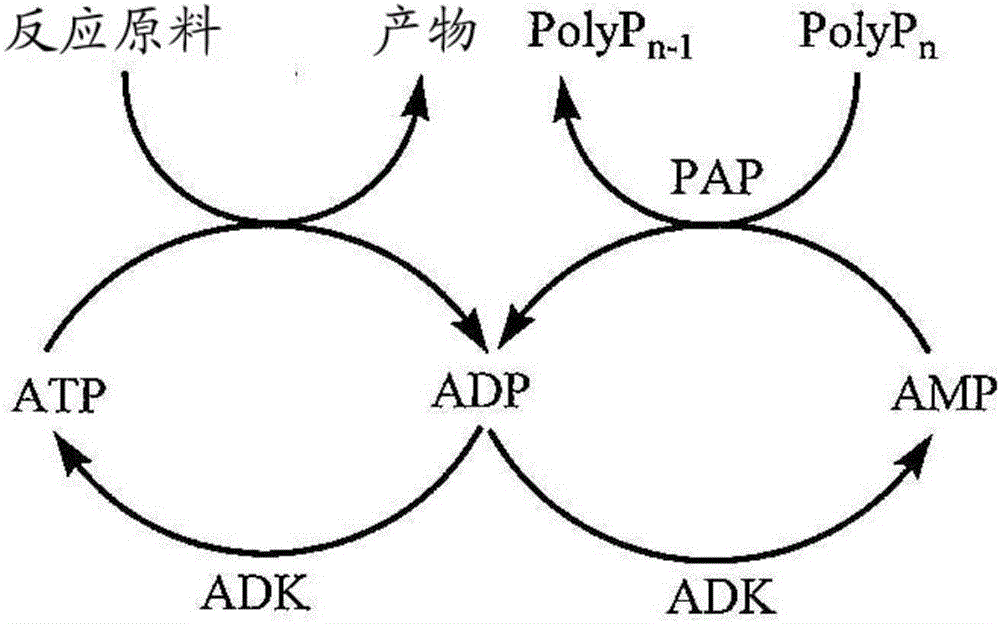
[0081] Glutathione synthetase (GSH II) that can be used in the present invention has the ability to recognize γ-Glu-Cys as a substrate and catalyze the generation of γ-Glu-Cys-Gly by bonding it to Gly in the presence of ATP As long as the reactive enzyme has the activity, its source, structure, etc. are not particularly limited. In the present invention, this activity is referred to as glutathione synthetase activity. 1 U of this activity refers to the activity of producing 1 μmol of glutathione in 1 minute at 30° C., and was measured under the following measurement conditions. In the present invention, the novel knowledge that glutathione synthase also has the ab...
Embodiment
[0166]
[0167] Preparation of γ-glutamylcysteine synthetase (GSH I) derived from Escherichia coli K12 strain
[0168]A DNA primer having a cleavage site and SD sequence bonded to the restriction enzyme SacI at the N-terminal portion of the GSH I gene (SEQ ID NO: 1) derived from the Escherichia coli K12 strain was prepared. A DNA primer (Primer-1: SEQ ID NO: 2) of the resulting sequence, a DNA primer (Primer-2: SEQ ID NO: 3) having a sequence in which a restriction enzyme KpnI cleavage site was bonded to the base sequence of the C-terminal part ). Using the DNA primers, the DNA between the sequences was amplified by PCR, thereby obtaining a DNA fragment containing the full length of the GSH I gene. At this time, the template used for PCR amplification is the genomic DNA of Escherichia coli K12 strain. The nucleotide sequence of the obtained DNA fragment was analyzed, and it was confirmed that it contained the full length of the GSH I gene (SEQ ID NO: 1). The obtained DN...
Embodiment 2
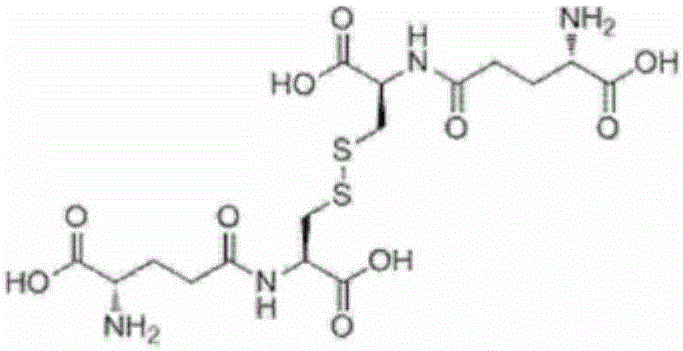
[0195] chemical formula 5
[0196]
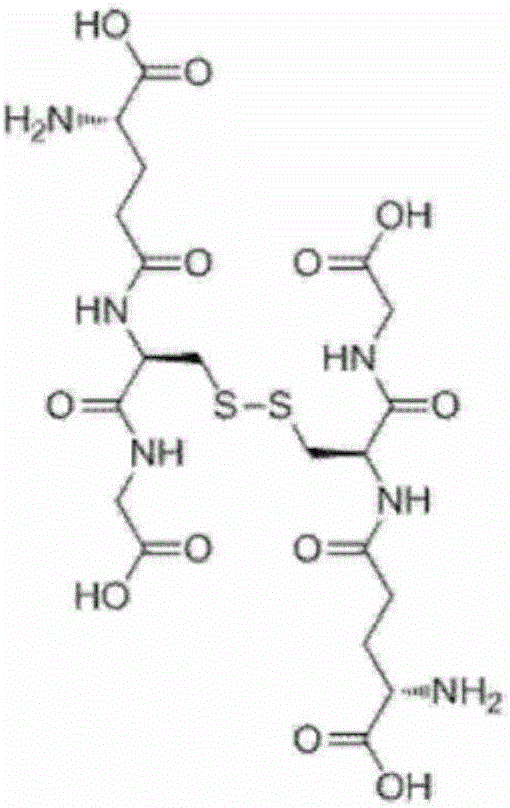
[0197] Add glycine 0.19g (2.53mmol), glutathione synthase (GSH II) enzyme solution 2g prepared in experiment 2, PAP enzyme solution prepared in experiment 4 in the reaction solution after the reaction of the above-mentioned embodiment 1 for 22 hours 2g, start the reaction. At this time, the pH was adjusted to 7.5 with 0.2 g of a 15% by mass sodium hydroxide aqueous solution. The reaction liquid was analyzed after 1 hour of reaction, and production of oxidized glutathione was confirmed. The yield was 20 mol% after 1 hour of reaction and 86 mol% after 6 hours of reaction relative to the initial L-cystine. In this case, the yield of reduced glutathione after the 6-hour reaction was 1 mol% or less relative to the initial L-cystine.
[0198] It should be noted that the GSH II enzyme solution prepared in Experiment 2 and the PAP enzyme solution prepared in Experiment 4 contained ADK derived from Escherichia coli used as host cells, so no add...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com