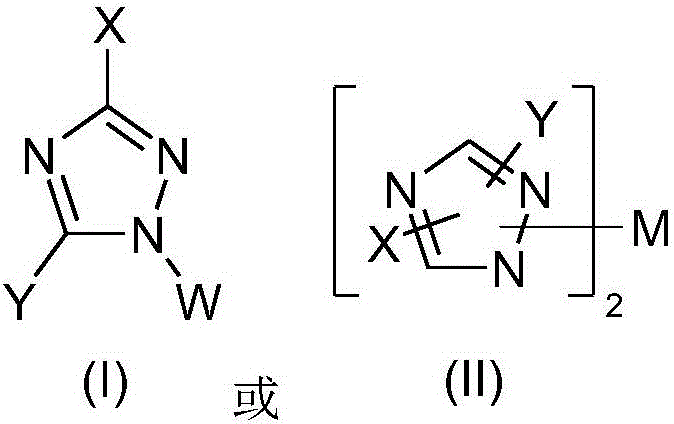
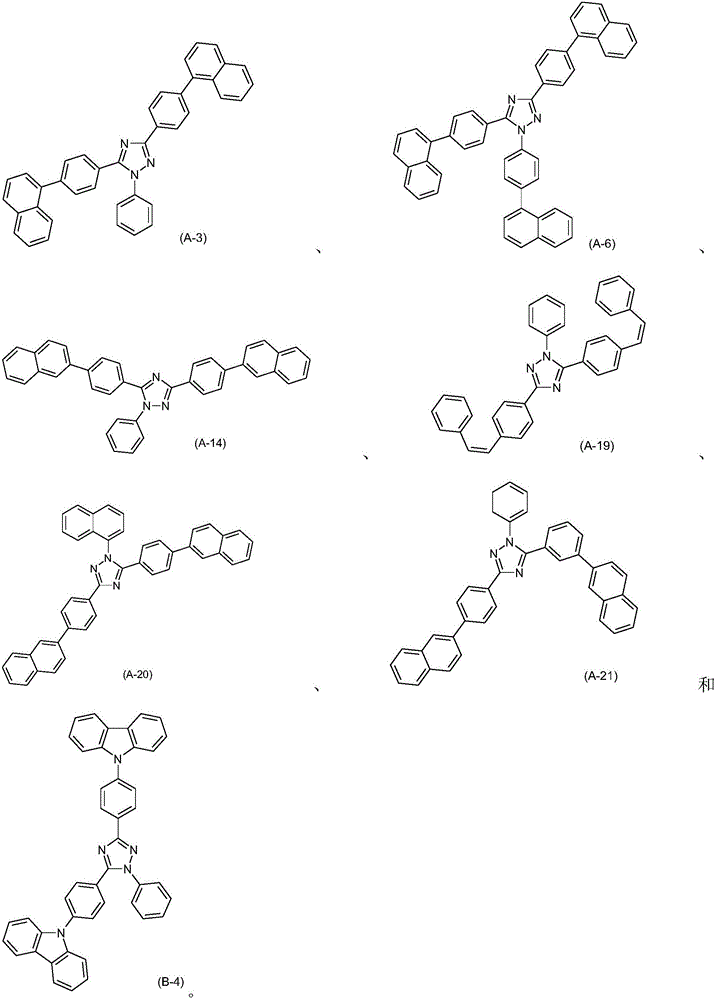
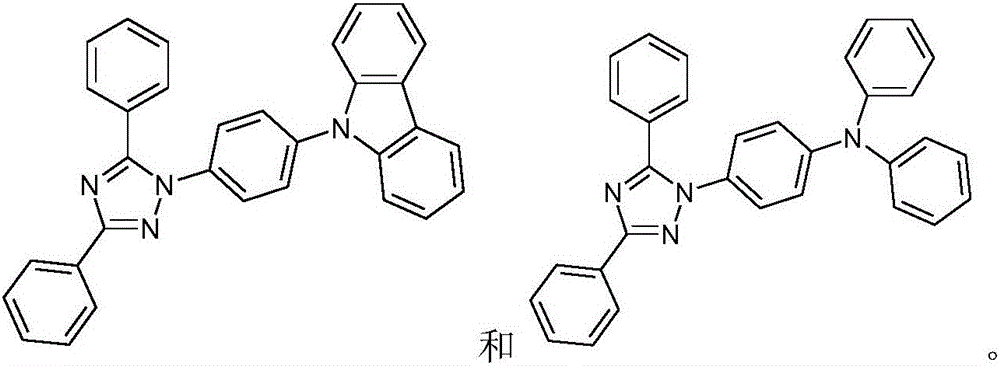
Fluorescent organic light emitting elements having high efficiency
A technology of organic light-emitting elements and light-emitting layers, which is used in organic semiconductor devices, photovoltaic power generation, electrical components, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0231]
[0232] a) 4-Bromobenzonitrile (56mmol) and ethanol (35g) were placed in a 100ml flask. Acetyl chloride (448 mmol) was added slowly while stirring. The mixture was stirred overnight (15 hours) at room temperature. After the reaction was completed, ethanol was removed by evaporation, and the residue was washed with diethyl ether. A white solid was obtained by drying in vacuo. (Yield: 84.4%) The product was used in the next reaction without further purification.
[0233]
[0234] b) The product from Example 1a (11.3 mmol), triethylamine (24.9 mmol), 4-bromobenzoyl chloride (11.3 mmol) and 35 ml of dichloromethane were placed in a 100 ml flask. The mixture was stirred overnight (15 hours) at -15°C to room temperature. After the reaction was complete, dichloromethane was removed by evaporation, and the residue was washed with warm tetrahydrofuran. A white solid was obtained by drying in vacuo. (crude material, yield: 105%). The product was used in the next rea...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com