Charge transfer type nano pharmaceutical composition and preparation method thereof
A technology of charge conversion and nano-medicine, applied in the field of medicine, can solve problems such as difficult to overcome the blood-brain barrier, and achieve the effect of overcoming the blood-brain barrier
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0032] The second embodiment of the present invention provides a method for preparing the above-mentioned charge conversion nano drug carrier, including:
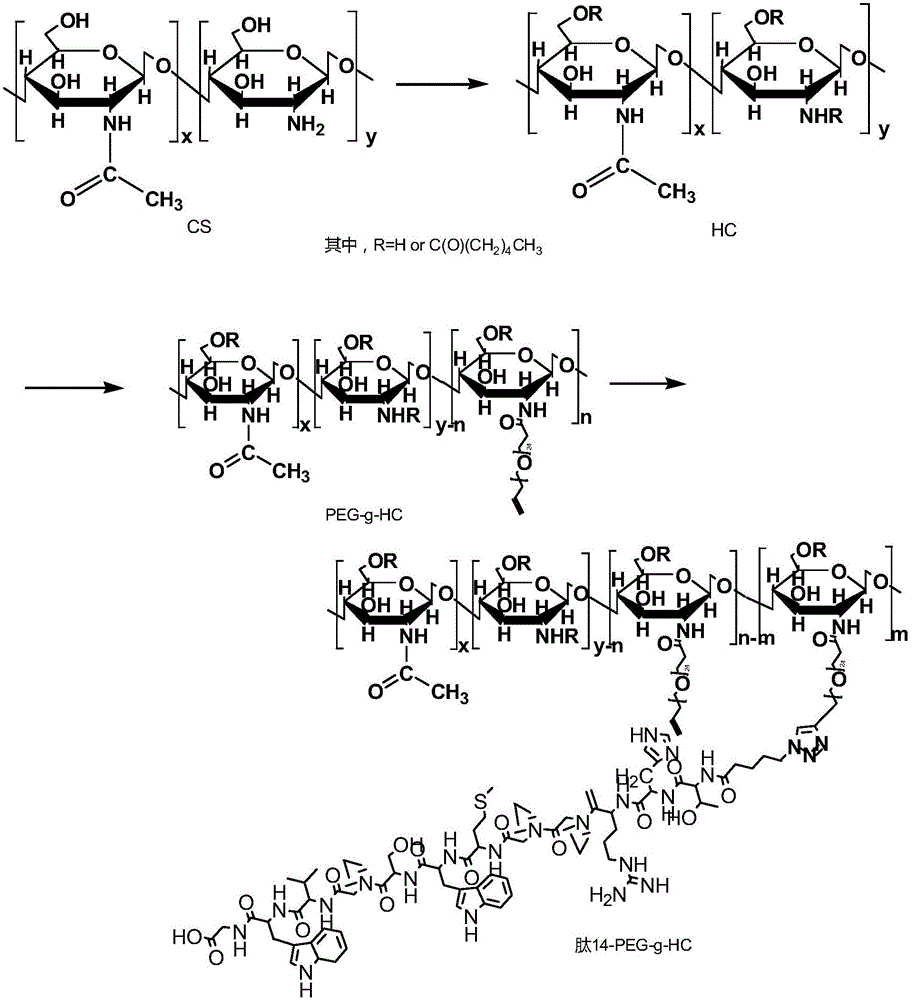
[0033] Carrier body preparation steps: acylate chitosan to generate hydrophobic chitosan, then graft polyethylene glycol with an azide-terminated targeting peptide through Click reaction to obtain a positively charged Targeting peptide modified polyethylene glycol-hydrophobic modified chitosan;
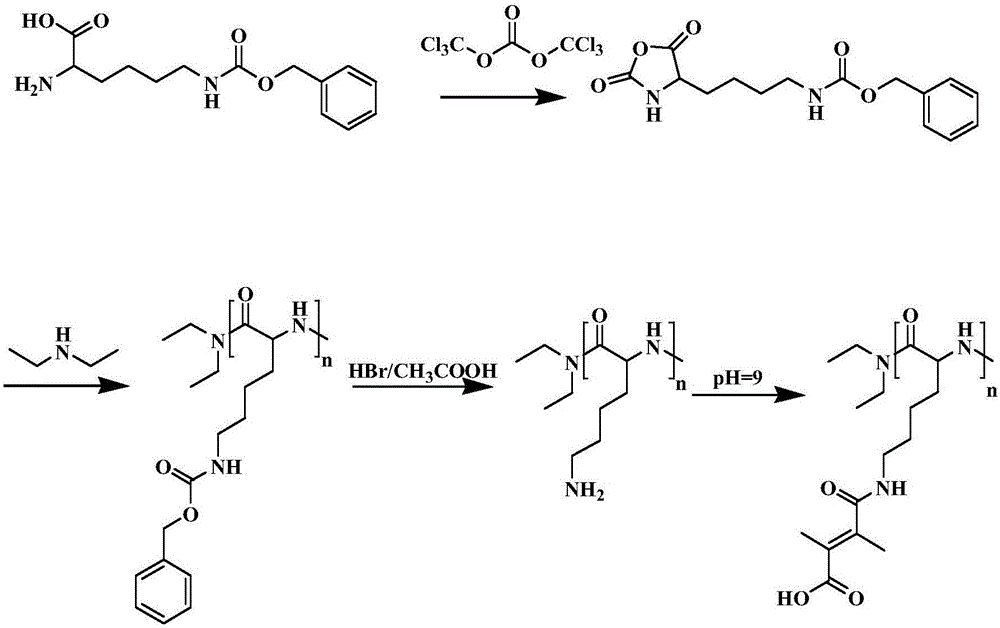
[0034] Carrier lock preparation steps: poly(Nε-benzyloxycarbonyllysine) debenzyloxy group to expose -NH 2 , and then react with 2,3-dimethylmaleic anhydride to obtain polylysine with side chain modification of 2,3-dimethylmaleic acid;
[0035]Nano drug particle carrier preparation steps: use the dialysis method, reverse evaporation method or emulsification solvent evaporation method to prepare the polyethylene glycol-hydrophobic modified chitosan modified by the targeting peptide into a nano core, and then use the positive and neg...
Embodiment 1
[0059] 1. Preparation of charge conversion drug carrier:
[0060] a. Synthesis of D-PLL-DMMA
[0061] Weigh 11.2g of Nε-benzyloxycarboxy-L-lysine solid and dissolve it in 180mL of anhydrous tetrahydrofuran; weigh 5.2g of bis(trichloromethyl)carbonate solid and dissolve it in 40mL of anhydrous tetrahydrofuran, pour into in the funnel. In the oil bath reaction, the reaction temperature was kept at about 50°C. Drop the dissolved bis(trichloromethyl)carbonate solution drop by drop into Nε-benzyloxycarboxy-L-lysine, drop it for 1-2 hours, and react for 0.5 hours after the drop is completed. The reaction product was concentrated by rotary evaporation to 20-30 mL. The product was precipitated with 200 mL of anhydrous n-butane, recrystallized at -4°C for 6 h and filtered with suction, and the filtrate was removed. The solid obtained by suction filtration was dried in a vacuum oven for 12 hours, and stored at -20°C;
[0062] In an ice-water bath, slowly drop 20 mg / mL diethylamine ...
Embodiment 2
[0073] 1. Preparation of charge conversion drug carrier:
[0074] a. Synthesis of D-PLL-DMMA
[0075] Weigh 11.2g of Nε-benzyloxycarboxy-L-lysine solid and dissolve it in 180mL of anhydrous tetrahydrofuran; weigh 5.2g of bis(trichloromethyl)carbonate solid and dissolve it in 40mL of anhydrous tetrahydrofuran, pour into in the funnel. In the oil bath reaction, the reaction temperature was kept at about 50°C. Drop the dissolved bis(trichloromethyl)carbonate solution drop by drop into Nε-benzyloxycarboxy-L-lysine, drop it for 1-2 hours, and react for 0.5 hours after the drop is completed. The reaction product was concentrated by rotary evaporation to 20-30 mL. The product was precipitated with 200 mL of anhydrous n-butane, recrystallized at -4°C for 6 h and filtered with suction, and the filtrate was removed. The solid obtained by suction filtration was dried in a vacuum oven for 12 hours, and stored at -20°C;
[0076] In an ice-water bath, slowly drop 20 mg / mL diethylamine ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com