Catalyst for continuously preparing 3,5-dimethyl piperidine and preparation method and application thereof
A technology of dimethylpiperidine and lutidine, which is applied in the field of catalytic hydrogenation of heterocyclic compounds, can solve the problems that the product yield cannot reach 99%, the cis-trans ratio is difficult to control, and the production efficiency is low. Effects of low cost, high catalytic activity and stability, and few by-products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
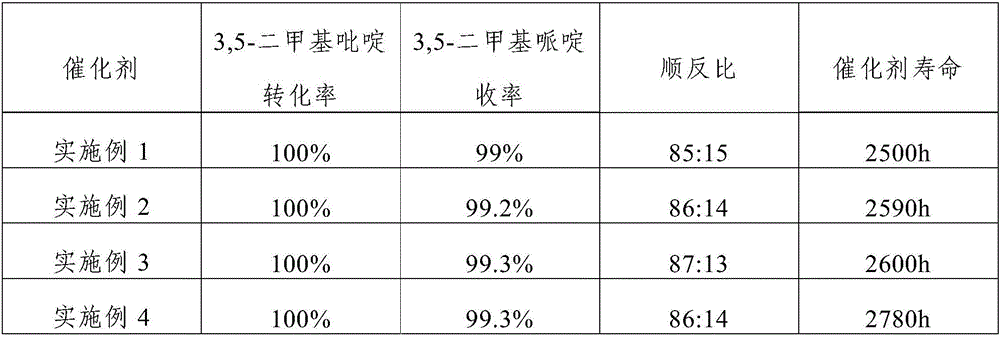
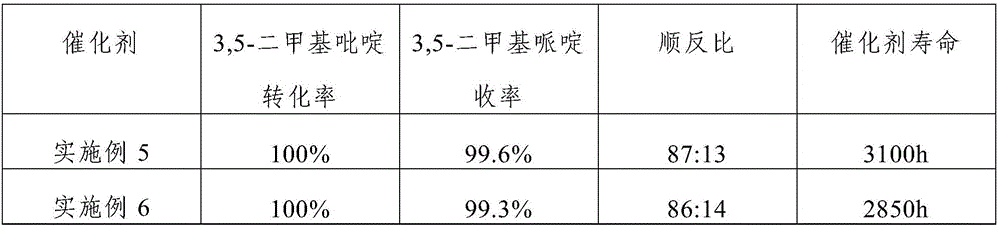
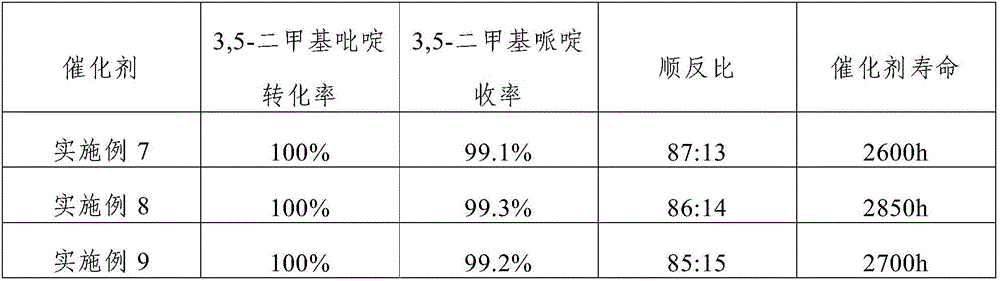
Embodiment 1
[0034] The catalyst of the present embodiment comprises activated carbon carrier, Ru, Rh and K loaded on the activated carbon carrier; the mass percentage composition of Ru is 1.5% in the described catalyst, the mass percentage composition of Rh is 1%, the mass percentage composition of K The content is 1.2%; the average particle diameter of the activated carbon carrier is 1.5mm, and the specific surface area is 920m 2 / g.
[0035] The preparation method of the catalyst of the present embodiment is:
[0036] Step 1, dissolving ruthenium trichloride containing 1.5g of ruthenium in dilute hydrochloric acid with a mass concentration of 2.5%, to obtain solution A;
[0037] Step 2, dissolving rhodium trichloride containing 1g of rhodium in dilute hydrochloric acid with a mass concentration of 3%, to obtain solution B;
[0038] Step 3. Mix the solution A described in step 1 and the solution B described in step 2 evenly to obtain solution C. Soak 96.3g of activated carbon carrier i...
Embodiment 2
[0044] The catalyst of the present embodiment includes activated carbon carrier, Ru, Rh and K loaded on the activated carbon carrier; the mass percentage of Ru in the catalyst is 2.5%, the mass percentage of Rh is 0.5%, and the mass percentage of K The content is 0.7%; the average particle diameter of the activated carbon carrier is 1.5mm, and the specific surface area is 1020m 2 / g.
[0045] The preparation method of the catalyst of the present embodiment is:
[0046] Step 1, dissolving ruthenium trichloride containing 2.5g ruthenium in dilute hydrochloric acid with a mass concentration of 3.5%, to obtain solution A;
[0047] Step 2, dissolving rhodium trichloride containing 0.5g rhodium in dilute hydrochloric acid with a mass concentration of 2.5%, to obtain solution B;
[0048] Step 3. Mix the solution A described in step 1 and the solution B described in step 2 evenly to obtain solution C. Soak 96.3g of activated carbon carrier in solution C, heat it to 40°C, keep it war...
Embodiment 3
[0054] The catalyst of the present embodiment includes activated carbon carrier, Ru, Rh and K loaded on the activated carbon carrier; the mass percentage of Ru in the catalyst is 5.0%, the mass percentage of Rh is 0.05%, and the mass percentage of K is The content is 0.1%; the average particle diameter of the activated carbon carrier is 2.5mm, and the specific surface area is 1200m 2 / g.
[0055] The preparation method of the catalyst of the present embodiment is:
[0056] Step 1, dissolving ruthenium trichloride containing 5.0 g of ruthenium in dilute hydrochloric acid with a mass concentration of 5%, to obtain solution A;
[0057] Step 2, dissolving rhodium trichloride containing 0.05g rhodium in dilute hydrochloric acid with a mass concentration of 2.5%, to obtain solution B;
[0058] Step 3. Mix the solution A described in step 1 and the solution B described in step 2 evenly to obtain solution C. Soak 94.85g of activated carbon carrier in solution C, heat it to 40°C, kee...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
| Specific surface area | aaaaa | aaaaa |
| Specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com