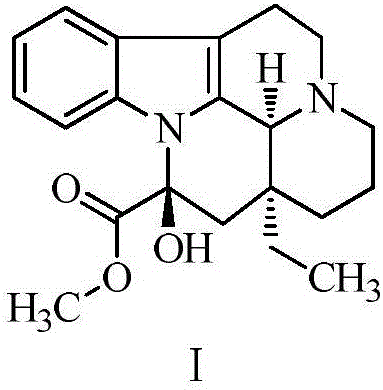
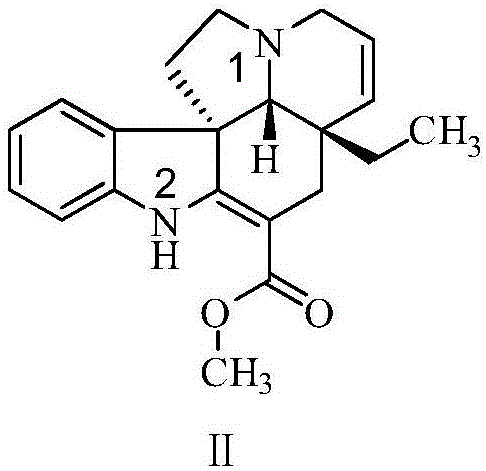
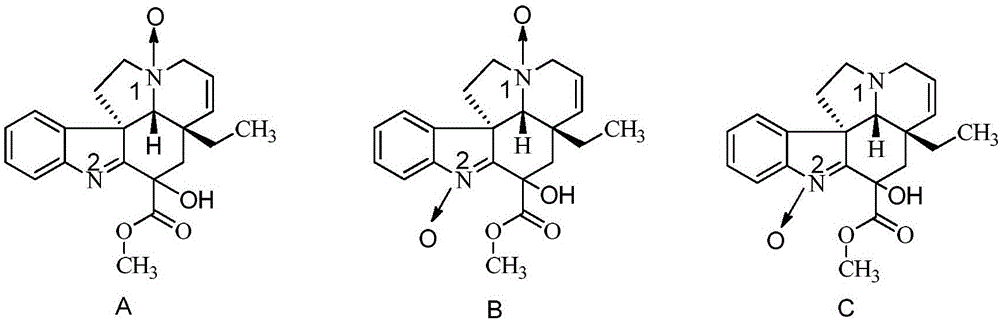
Refining method for vincamine
A purification method and vincamine technology, applied in the direction of organic chemistry and the like, can solve the problems of difficult separation and removal, affecting the quality of vincamine, etc., and achieve the effects of easy operation, good removal effect and high safety.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Step 1: Add 12.0g of vincamine crude product and 60ml of anhydrous organic solvent to a 100ml single-necked bottle. The selection of the organic solvent is shown in Table 1. Under stirring, the temperature is raised to 60°C for beating for 7 hours, then down to 25°C and stirred for 8 hours. Then lower it to 0°C for crystallization for 4 hours, filter with suction, wash the filter cake with 12 ml of cold methanol, and dry at 40°C for 6-9 hours. Obtain white vincamine beating product, N-oxide impurity, purity and yield situation are shown in Table 1.
[0034] Step 2: Add 6.0 g of vincamine slurry and 200 ml of dichloromethane into a 500 ml single-necked bottle, dissolve under stirring, filter, and concentrate the filtrate under reduced pressure to obtain a concentrate.
[0035]Step 3: Add the concentrate to a 250ml three-necked bottle, add 10ml of dichloromethane and 20ml of methanol, heat to 60°C for beating for 3 hours, lower to 21°C and stir for 7 hours, a large amount...
Embodiment 2
[0039] Step 1: Add 12.0g of crude vincamine and 60ml of N,N-dimethylformamide to a 100ml single-necked bottle, heat up to a certain temperature and beat for 7 hours while stirring. The selection of the temperature is shown in Table 2, and the temperature is lowered to 25°C Stir for 8 hours, then drop to 0°C to crystallize for 4 hours, filter with suction, wash the filter cake with 12ml of cold methanol, and dry at 40°C for 6-9 hours. Obtain white vincamine beating, N-oxide impurities, purity and yield are shown in Table 2.
[0040] Step 2: Add 6.0 g of vincamine slurry and 200 ml of dichloromethane into a 500 ml single-necked bottle, dissolve under stirring, filter, and concentrate the filtrate under reduced pressure to obtain a concentrate.
[0041] Step 3: Add the concentrate to a 250ml three-necked bottle, add 10ml of dichloromethane and 20ml of methanol, heat to 60°C for beating for 3 hours, lower to 21°C and stir for 7 hours, a large amount of white crystals are precipita...
Embodiment 3
[0045] Step 1: Add 12.0g of crude vincamine and 60ml of N,N-dimethylformamide to a 100ml single-necked bottle, raise the temperature to 60°C for 7 hours under stirring, lower the temperature to 25°C and stir for 8 hours, and then lower the temperature to 0°C for analysis After crystallization for 4 hours, filter with suction, wash the filter cake with 12ml of cold methanol, and dry at 40°C for 6-9 hours. 10.4 g of white vincamine slurry was obtained, with a molar yield of 87.0%.
[0046] Step 2: Add 6.0 g of vincamine slurry and 200 ml of organic solvent into a 500 ml single-necked bottle. The selection of the organic solvent is shown in Table 3. Dissolve under stirring, filter, and concentrate the filtrate under reduced pressure to obtain a concentrate.
[0047] Step 3: Add the concentrate to a 250ml three-neck bottle, add 10ml of dichloromethane and 20ml of methanol mixed solvent, heat to 60°C for beating for 3 hours, lower to 21°C and stir for 7 hours, filter with suction, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com